Rapid and tunable control of protein stability in Caenorhabditis elegans using a small molecule
- PMID: 23991108
- PMCID: PMC3750007
- DOI: 10.1371/journal.pone.0072393
Rapid and tunable control of protein stability in Caenorhabditis elegans using a small molecule
Abstract
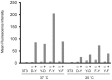
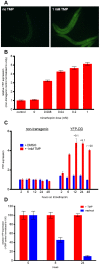
Destabilizing domains are conditionally unstable protein domains that can be fused to a protein of interest resulting in degradation of the fusion protein in the absence of stabilizing ligand. These engineered protein domains enable rapid, reversible and dose-dependent control of protein expression levels in cultured cells and in vivo. To broaden the scope of this technology, we have engineered new destabilizing domains that perform well at temperatures of 20-25°C. This raises the possibility that our technology could be adapted for use at any temperature. We further show that these new destabilizing domains can be used to regulate protein concentrations in C. elegans. These data reinforce that DD can function in virtually any organism and temperature.
Conflict of interest statement
Figures


Similar articles
-
A general chemical method to regulate protein stability in the mammalian central nervous system.Chem Biol. 2010 Sep 24;17(9):981-8. doi: 10.1016/j.chembiol.2010.07.009. Chem Biol. 2010. PMID: 20851347 Free PMC article.
-
Regulation of lead toxicity by heat shock protein 90 (daf-21) is affected by temperature in Caenorhabditis elegans.Ecotoxicol Environ Saf. 2014 Jun;104:317-22. doi: 10.1016/j.ecoenv.2014.03.016. Epub 2014 Apr 15. Ecotoxicol Environ Saf. 2014. PMID: 24726945
-
Using Caenorhabditis elegans as a model system to study protein homeostasis in a multicellular organism.J Vis Exp. 2013 Dec 18;(82):e50840. doi: 10.3791/50840. J Vis Exp. 2013. PMID: 24378578 Free PMC article.
-
Genetic analysis of RGS protein function in Caenorhabditis elegans.Methods Enzymol. 2004;389:305-20. doi: 10.1016/S0076-6879(04)89018-9. Methods Enzymol. 2004. PMID: 15313573 Review.
-
Principles of PAR polarity in Caenorhabditis elegans embryos.Nat Rev Mol Cell Biol. 2013 May;14(5):315-22. doi: 10.1038/nrm3558. Epub 2013 Apr 18. Nat Rev Mol Cell Biol. 2013. PMID: 23594951 Review.
Cited by
-
Prey for the Proteasome: Targeted Protein Degradation-A Medicinal Chemist's Perspective.Angew Chem Int Ed Engl. 2020 Sep 1;59(36):15448-15466. doi: 10.1002/anie.202004310. Epub 2020 Jul 30. Angew Chem Int Ed Engl. 2020. PMID: 32428344 Free PMC article. Review.
-
Engineering living therapeutics with synthetic biology.Nat Rev Drug Discov. 2021 Dec;20(12):941-960. doi: 10.1038/s41573-021-00285-3. Epub 2021 Oct 6. Nat Rev Drug Discov. 2021. PMID: 34616030 Review.
-
Protocol for In Vivo Evaluation and Use of Destabilizing Domains in the Eye, Liver, and Beyond.STAR Protoc. 2020 Sep 18;1(2):100094. doi: 10.1016/j.xpro.2020.100094. Epub 2020 Aug 27. STAR Protoc. 2020. PMID: 32995756 Free PMC article.
-
Emerging Chemistry Strategies for Engineering Native Chromatin.J Am Chem Soc. 2017 Jul 12;139(27):9090-9096. doi: 10.1021/jacs.7b03430. Epub 2017 Jun 27. J Am Chem Soc. 2017. PMID: 28635271 Free PMC article.
-
Development of a New DHFR-Based Destabilizing Domain with Enhanced Basal Turnover and Applicability in Mammalian Systems.ACS Chem Biol. 2022 Oct 21;17(10):2877-2889. doi: 10.1021/acschembio.2c00518. Epub 2022 Sep 19. ACS Chem Biol. 2022. PMID: 36122928 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

