Carboplatin +/- topotecan ophthalmic artery chemosurgery for intraocular retinoblastoma
- PMID: 23991112
- PMCID: PMC3749169
- DOI: 10.1371/journal.pone.0072441
Carboplatin +/- topotecan ophthalmic artery chemosurgery for intraocular retinoblastoma
Abstract
Purpose: Carboplatin administered systemically or periocularly can result in dramatic and prompt regression of retinoblastoma. However, both routes are rarely curative alone and have undesirable side effects. We aimed to assess the efficacy and toxicity of carboplatin +/- topotecan delivered by ophthalmic artery chemosurgery whereby chemotherapy is infused into the eye via the ophthalmic artery.
Methods: This retrospective, IRB-approved study investigated retinoblastoma patients whom received carboplatin +/- topotecan ophthalmic artery chemosurgery. Patient survival, ocular survival, hematologic toxicity, ocular toxicity, second cancer development and electroretinogram response were all evaluated.
Results: 57 carboplatin +/- topotecan infusions (of 111 total) were performed in 31 eyes of 24 patients. The remaining infusions were melphalan-containing. All patients were alive and no patient developed a second malignancy at a median follow up of 25 months. The Kaplan-Meier estimate of ocular survival at two years was 89.9% (95% confidence interval [CI], 82.1-97.9%) for all eyes. Grade 3 or 4 neutropenia developed in two patients and one patient developed metastatic disease. By univariate analysis, neither increasing maximum carboplatin/topotecan dose nor cumulative carboplatin/topotecan dose was associated with statistically significant reduction in the electroretinogram responses.
Conclusion: Carboplatin +/- topotecan infusions are effective for ophthalmic artery chemosurgery in retinoblastoma: they demonstrate low hematologic and ocular toxicity and no statistically significant influence on electroretinogram responses, and used in conjunction with melphalan-containing OAC, demonstrate excellent patient survival and satisfactory ocular survival.
Conflict of interest statement
Figures
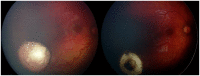

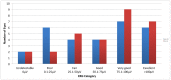
References
-
- Abramson DH, Ellsworth RM, Zimmerman LE (1976) Nonocular cancer in retinoblastoma survivors. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 81: 454–7. - PubMed
-
- Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, et al. (1993) Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst 85: 1121–8. - PubMed
-
- Abramson DH, Frank CM (1998) Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology 105: 573–80. - PubMed
-
- Marees T, Moll AC, Imhof SM, de Boer MR, Ringens PJ, et al. (2008) Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst 100: 1771–9. - PubMed
-
- Kupfer C (1953) Retinoblastoma treated with intravenous nitrogen mustard. Am J Ophthalmol 36: 1721–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

