Deep brain stimulation imposes complex informational lesions
- PMID: 23991221
- PMCID: PMC3753277
- DOI: 10.1371/journal.pone.0074462
Deep brain stimulation imposes complex informational lesions
Abstract
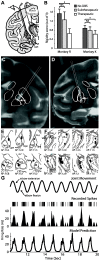
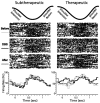
Deep brain stimulation (DBS) therapy has become an essential tool for treating a range of brain disorders. In the resting state, DBS is known to regularize spike activity in and downstream of the stimulated brain target, which in turn has been hypothesized to create informational lesions. Here, we specifically test this hypothesis using repetitive joint articulations in two non-human Primates while recording single-unit activity in the sensorimotor globus pallidus and motor thalamus before, during, and after DBS in the globus pallidus (GP) GP-DBS resulted in: (1) stimulus-entrained firing patterns in globus pallidus, (2) a monophasic stimulus-entrained firing pattern in motor thalamus, and (3) a complete or partial loss of responsiveness to joint position, velocity, or acceleration in globus pallidus (75%, 12/16 cells) and in the pallidal receiving area of motor thalamus (ventralis lateralis pars oralis, VLo) (38%, 21/55 cells). Despite loss of kinematic tuning, cells in the globus pallidus (63%, 10/16 cells) and VLo (84%, 46/55 cells) still responded to one or more aspects of joint movement during GP-DBS. Further, modulated kinematic tuning did not always necessitate modulation in firing patterns (2/12 cells in globus pallidus; 13/23 cells in VLo), and regularized firing patterns did not always correspond to altered responses to joint articulation (3/4 cells in globus pallidus, 11/33 cells in VLo). In this context, DBS therapy appears to function as an amalgam of network modulating and network lesioning therapies.
Conflict of interest statement
Figures




References
-
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C et al. (1998) Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 339: 1105-1111. doi:10.1056/NEJM199810153391603. PubMed: 9770557. - DOI - PubMed
-
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM et al. (1991) Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337: 403-406. doi:10.1016/0140-6736(91)91175-T. PubMed: 1671433. - DOI - PubMed
-
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D et al. (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45: 651-660. doi:10.1016/j.neuron.2005.02.014. PubMed: 15748841. - DOI - PubMed
-
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R et al. (2010) A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 68: 521-534. doi:10.1002/ana.22089. PubMed: 20687206. - DOI - PubMed
-
- Fisher R, Salanova V, Witt T, Worth R, Henry T et al. (2010) Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51: 899-908. doi:10.1111/j.1528-1167.2010.02536.x. PubMed: 20331461. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

