A family of fluoride-specific ion channels with dual-topology architecture
- PMID: 23991286
- PMCID: PMC3755343
- DOI: 10.7554/eLife.01084
A family of fluoride-specific ion channels with dual-topology architecture
Erratum in
- Elife. 2013;2:e01700
Abstract
Fluoride ion, ubiquitous in soil, water, and marine environments, is a chronic threat to microorganisms. Many prokaryotes, archea, unicellular eukaryotes, and plants use a recently discovered family of F(-) exporter proteins to lower cytoplasmic F(-) levels to counteract the anion's toxicity. We show here that these 'Fluc' proteins, purified and reconstituted in liposomes and planar phospholipid bilayers, form constitutively open anion channels with extreme selectivity for F(-) over Cl(-). The active channel is a dimer of identical or homologous subunits arranged in antiparallel transmembrane orientation. This dual-topology assembly has not previously been seen in ion channels but is known in multidrug transporters of the SMR family, and is suggestive of an evolutionary antecedent of the inverted repeats found within the subunits of many membrane transport proteins. DOI:http://dx.doi.org/10.7554/eLife.01084.001.
Keywords: E. coli; fluoride; ion channel; membrane topology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





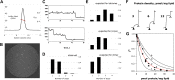
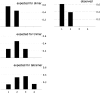
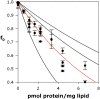
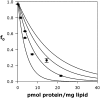

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

