Are reactive oxygen species always detrimental to pathogens?
- PMID: 23992156
- PMCID: PMC3924804
- DOI: 10.1089/ars.2013.5447
Are reactive oxygen species always detrimental to pathogens?
Abstract
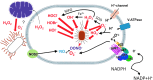
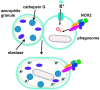
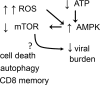
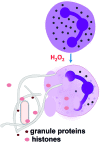
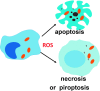
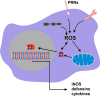
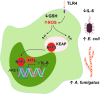
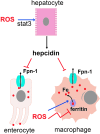
Reactive oxygen species (ROS) are deadly weapons used by phagocytes and other cell types, such as lung epithelial cells, against pathogens. ROS can kill pathogens directly by causing oxidative damage to biocompounds or indirectly by stimulating pathogen elimination by various nonoxidative mechanisms, including pattern recognition receptors signaling, autophagy, neutrophil extracellular trap formation, and T-lymphocyte responses. Thus, one should expect that the inhibition of ROS production promote infection. Increasing evidences support that in certain particular infections, antioxidants decrease and prooxidants increase pathogen burden. In this study, we review the classic infections that are controlled by ROS and the cases in which ROS appear as promoters of infection, challenging the paradigm. We discuss the possible mechanisms by which ROS could promote particular infections. These mechanisms are still not completely clear but include the metabolic effects of ROS on pathogen physiology, ROS-induced damage to the immune system, and ROS-induced activation of immune defense mechanisms that are subsequently hijacked by particular pathogens to act against more effective microbicidal mechanisms of the immune system. The effective use of antioxidants as therapeutic agents against certain infections is a realistic possibility that is beginning to be applied against viruses.
Figures



References
-
- Albert ML, Sauter B, and Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392: 86–89, 1998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

