Increased L-DOPA-derived dopamine following selective MAO-A or -B inhibition in rat striatum depleted of dopaminergic and serotonergic innervation
- PMID: 23992249
- PMCID: PMC3949649
- DOI: 10.1111/bph.12349
Increased L-DOPA-derived dopamine following selective MAO-A or -B inhibition in rat striatum depleted of dopaminergic and serotonergic innervation
Abstract
Background and purpose: Selective MAO type B (MAO-B) inhibitors are effective in potentiation of the clinical effect of L-DOPA in Parkinson's disease, but dopamine (DA) is deaminated mainly by MAO type A (MAO-A) in rat brain. We sought to clarify the roles of MAO-A and MAO-B in deamination of DA formed from exogenous L-DOPA in rat striatum depleted of dopaminergic, or both dopaminergic and serotonergic innervations. We also studied the effect of organic cation transporter-3 (OCT-3) inhibition by decinium-22 on extracellular DA levels following L-DOPA.
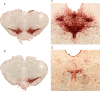
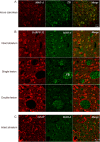
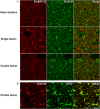
Experimental approach: Striatal dopaminergic and/or serotonergic neuronal innervations were lesioned by 6-hydroxydopamine or 5,7-dihydroxytryptamine respectively. Microdialysate DA levels after systemic L-DOPA were measured after inhibition of MAO-A or MAO-B by clorgyline or rasagiline respectively. MAO subtype localization in the striatum was determined by immunofluorescence.
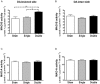
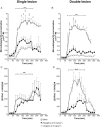
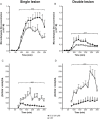
Key results: Rasagiline increased DA extracellular levels following L-DOPA to a greater extent in double- than in single-lesioned rats (2.8- and 1.8-fold increase, respectively, relative to saline treatment); however, clorgyline elevated DA levels in both models over 10-fold. MAO-A was strongly expressed in medium spiny neurons (MSNs) in intact and lesioned striata, while MAO-B was localized in glia and to a small extent in MSNs. Inhibition of OCT-3 increased DA levels in the double- more than the single-lesion animals.
Conclusions and implications: In striatum devoid of dopaminergic and serotonergic inputs, most deamination of L-DOPA-derived DA is mediated by MAO-A in MSN and a smaller amount by MAO-B in both MSN and glia. OCT-3 plays a significant role in uptake of DA from extracellular space. Inhibitors of OCT-3 are potential future targets for anti-Parkinsonian treatments.
Keywords: 6-hydroxydopamine; L-DOPA; OCT-3; Parkinson's disease; clorgyline; glial cells; medium spiny neurons; microdialysis; rasagiline.
© 2013 The British Pharmacological Society.
Figures

References
-
- Arai R, Horiike K, Hasegawa Y. Dopamine-degrading activity of monoamine oxidase is not detected by histochemistry in neurons of the substantia nigra pars compacta of the rat. Brain Res. 1998;812:275–278. - PubMed
-
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. J Neurochem. 1990;55:981–988. - PubMed
-
- Carlsson A, Fowler CJ, Magnusson T, Oreland L, Wiberg A. The activities of monoamine oxidase-A and-B, succinate dehydrogenase and acid phosphatase in the rat brain after hemitransection. Naunyn Schmiedebergs Arch Pharmacol. 1981;316:51–55. - PubMed
-
- Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in Parkinsonian rats. Brain. 2007;130(Pt 7):1819–1833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

