The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction
- PMID: 23992980
- PMCID: PMC3809823
- DOI: 10.1016/j.biomaterials.2013.08.017
The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction
Abstract
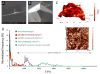
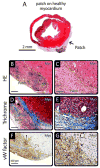
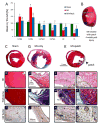
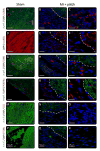
Regeneration of the damaged myocardium is one of the most challenging fronts in the field of tissue engineering due to the limited capacity of adult heart tissue to heal and to the mechanical and structural constraints of the cardiac tissue. In this study we demonstrate that an engineered acellular scaffold comprising type I collagen, endowed with specific physiomechanical properties, improves cardiac function when used as a cardiac patch following myocardial infarction. Patches were grafted onto the infarcted myocardium in adult murine hearts immediately after ligation of left anterior descending artery and the physiological outcomes were monitored by echocardiography, and by hemodynamic and histological analyses four weeks post infarction. In comparison to infarcted hearts with no treatment, hearts bearing patches preserved contractility and significantly protected the cardiac tissue from injury at the anatomical and functional levels. This improvement was accompanied by attenuated left ventricular remodeling, diminished fibrosis, and formation of a network of interconnected blood vessels within the infarct. Histological and immunostaining confirmed integration of the patch with native cardiac cells including fibroblasts, smooth muscle cells, epicardial cells, and immature cardiomyocytes. In summary, an acellular biomaterial with specific biomechanical properties promotes the endogenous capacity of the infarcted myocardium to attenuate remodeling and improve heart function following myocardial infarction.
Keywords: Angiogenesis; Cardiac tissue engineering; Cardiomyocyte; Collagen; Heart; Scaffold.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Cardiac patching and the regeneration of infarcted myocardium: where do we go from here?Future Cardiol. 2014 Mar;10(2):167-70. doi: 10.2217/fca.13.101. Future Cardiol. 2014. PMID: 24762242
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

