Allosteric activation of functionally asymmetric RAF kinase dimers
- PMID: 23993095
- PMCID: PMC3844432
- DOI: 10.1016/j.cell.2013.07.046
Allosteric activation of functionally asymmetric RAF kinase dimers
Abstract
Although RAF kinases are critical for controlling cell growth, their mechanism of activation is incompletely understood. Recently, dimerization was shown to be important for activation. Here we show that the dimer is functionally asymmetric with one kinase functioning as an activator to stimulate activity of the partner, receiver kinase. The activator kinase did not require kinase activity but did require N-terminal phosphorylation that functioned allosterically to induce cis-autophosphorylation of the receiver kinase. Based on modeling of the hydrophobic spine assembly, we also engineered a constitutively active mutant that was independent of Ras, dimerization, and activation-loop phosphorylation. As N-terminal phosphorylation of BRAF is constitutive, BRAF initially functions to activate CRAF. N-terminal phosphorylation of CRAF was dependent on MEK, suggesting a feedback mechanism and explaining a key difference between BRAF and CRAF. Our work illuminates distinct steps in RAF activation that function to assemble the active conformation of the RAF kinase.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
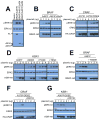

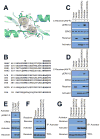
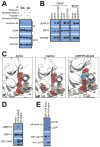
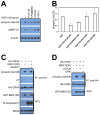

References
-
- Baccarini M. Second nature: biological functions of the Raf-1 “kinase”. FEBS Lett. 2005;579:3271–3277. - PubMed
-
- Baljuls A, Mueller T, Drexler HC, Hekman M, Rapp UR. Unique N-region determines low basal activity and limited inducibility of A-RAF kinase: the role of N-region in the evolutionary divergence of RAF kinase function in vertebrates. J Biol Chem. 2007;282:26575–26590. - PubMed
-
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. - PubMed
-
- Brennan DF, Dar AC, Hertz NT, Chao WC, Burlingame AL, Shokat KM, Barford D. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature. 2011;472:366–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

