A comparison of two probiotic strains of bifidobacteria in premature infants
- PMID: 23993139
- PMCID: PMC3842430
- DOI: 10.1016/j.jpeds.2013.07.017
A comparison of two probiotic strains of bifidobacteria in premature infants
Abstract
Objective: To determine the impact of 2 probiotic bifidobacteria on the fecal microbiota of premature infants fed either human milk or formula.
Study design: In the first of two phase 1 clinical trials, 12 premature infants receiving formula feedings were assigned randomly to receive either Bifidobacterium longum ssp infantis or Bifidobacterium animalis ssp lactis in increasing doses during a 5-week period. In the second, 9 premature infants receiving their mother's milk received each of the two bifidobacteria for 2 weeks separated by a 1-week washout period. Serial stool specimens from each infant were analyzed by terminal restriction fragment-length polymorphism and quantitative polymerase chain reaction for bacterial composition.
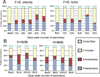
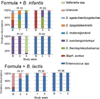
Results: Among the formula-fed infants, there was a greater increase in fecal bifidobacteria among infants receiving B infantis (Binf) than those receiving B lactis (Blac). This difference was most marked at a dose of 1.4 × 10(9) colony-forming units twice daily (P < .05). Bacterial diversity improved over dose/time in those infants receiving Binf. Among the human milk-fed infants, greater increases in fecal bifidobacteria and decreases in γ-Proteobacteria followed the administration of Binf than Blac. The B longum group (which includes Binf but not Blac) was the dominant bifidobacteria among the human milk-fed infants, regardless of the probiotic administered.
Conclusions: Binf was more effective at colonizing the fecal microbiota than Blac in both formula-fed and human milk-fed premature infants. The combination of human milk plus Binf resulted in the greatest fecal levels of bifidobacteria.
Trial registration: ClinicalTrials.gov NCT00810160.
Keywords: B infantis; B lactis; Bac-TRFLP; Bacilli-specific terminal restriction fragment-length polymorphism; Bif-TRFLP; Bifidobacteria-specific terminal restriction fragment-length polymorphism; Binf; Blac; Colony-forming units; F+Binf; F+Blac; Formula plus Binf; Formula plus Blac; H+Binf; H+Blac; HMO; Human milk oligosaccharides; Human milk plus Binf; Human milk plus Blac; NEC; Necrotizing enterocolitis; PBS; PCoA; Phosphate-buffered saline; Principal coordinate analysis; Quantitative polymerase chain reaction; Ribosomal RNA; TRFLP; Terminal restriction fragment-length polymorphism; UC Davis; University of California, Davis; WO; Washout; cfu; qPCR; rRNA.
Copyright © 2013 Mosby, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg. 2012;47:241–248. - PubMed
-
- Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125:921–930. - PubMed
-
- Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol Nutr Food Res. 2007;51:1398–1405. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

