Retinoid signaling in progenitors controls specification and regeneration of the urothelium
- PMID: 23993789
- PMCID: PMC4024836
- DOI: 10.1016/j.devcel.2013.07.017
Retinoid signaling in progenitors controls specification and regeneration of the urothelium
Abstract
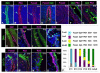
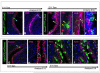
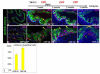
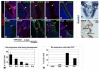
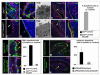
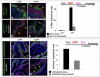
The urothelium is a multilayered epithelium that serves as a barrier between the urinary tract and blood, preventing the exchange of water and toxic substances. It consists of superficial cells specialized for synthesis and transport of uroplakins that assemble into a tough apical plaque, one or more layers of intermediate cells, and keratin 5-expressing basal cells (K5-BCs), which are considered to be progenitors in the urothelium and other specialized epithelia. Fate mapping, however, reveals that intermediate cells rather than K5-BCs are progenitors in the adult regenerating urothelium, that P cells, a transient population, are progenitors in the embryo, and that retinoids are critical in P cells and intermediate cells, respectively, for their specification during development and regeneration. These observations have important implications for tissue engineering and repair and, ultimately, may lead to treatments that prevent loss of the urothelial barrier, a major cause of voiding dysfunction and bladder pain syndrome.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Blumberg B, Bolado J, Jr., Moreno TA, Kintner C, Evans RM, Papalopulu N. An essential role for retinoid signaling in anteroposterior neural patterning. Development. 1997;124:373–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
