Methods to detect, characterize, and remove motion artifact in resting state fMRI
- PMID: 23994314
- PMCID: PMC3849338
- DOI: 10.1016/j.neuroimage.2013.08.048
Methods to detect, characterize, and remove motion artifact in resting state fMRI
Abstract
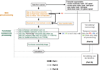
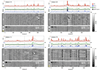
Head motion systematically alters correlations in resting state functional connectivity fMRI (RSFC). In this report we examine impact of motion on signal intensity and RSFC correlations. We find that motion-induced signal changes (1) are often complex and variable waveforms, (2) are often shared across nearly all brain voxels, and (3) often persist more than 10s after motion ceases. These signal changes, both during and after motion, increase observed RSFC correlations in a distance-dependent manner. Motion-related signal changes are not removed by a variety of motion-based regressors, but are effectively reduced by global signal regression. We link several measures of data quality to motion, changes in signal intensity, and changes in RSFC correlations. We demonstrate that improvements in data quality measures during processing may represent cosmetic improvements rather than true correction of the data. We demonstrate a within-subject, censoring-based artifact removal strategy based on volume censoring that reduces group differences due to motion to chance levels. We note conditions under which group-level regressions do and do not correct motion-related effects.
Keywords: Artifact; Functional connectivity; MRI; Motion; Movement; Resting state.
© 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures



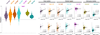









References
-
- Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. NeuroImage. 2013;76:436–438. - PubMed
-
- Chen G, Chen G, Xie C, Ward BD, Li W, Antuono P, Li S-J. A method to determine the necessity for global signal regression in resting-state fMRI studies. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2012;68:1828–1835. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P30 HD062171/HD/NICHD NIH HHS/United States
- F30 MH100872/MH/NIMH NIH HHS/United States
- K12 EY016336/EY/NEI NIH HHS/United States
- 5R01HD057076-03-S1/HD/NICHD NIH HHS/United States
- F30 MH094032/MH/NIMH NIH HHS/United States
- R01HD057076/HD/NICHD NIH HHS/United States
- R21 NS061144/NS/NINDS NIH HHS/United States
- U54MH091657/MH/NIMH NIH HHS/United States
- F30 MH940322/MH/NIMH NIH HHS/United States
- P50 NS006833/NS/NINDS NIH HHS/United States
- R21NS061144/NS/NINDS NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- P30 NS048056/NS/NINDS NIH HHS/United States
- K12 EY16336/EY/NEI NIH HHS/United States
- R01 HD057076/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

