Neutrophil cell surface receptors and their intracellular signal transduction pathways
- PMID: 23994464
- PMCID: PMC3827506
- DOI: 10.1016/j.intimp.2013.06.034
Neutrophil cell surface receptors and their intracellular signal transduction pathways
Abstract
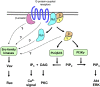
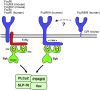
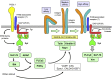
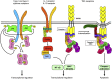
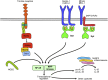
Neutrophils play a critical role in the host defense against bacterial and fungal infections, but their inappropriate activation also contributes to tissue damage during autoimmune and inflammatory diseases. Neutrophils express a large number of cell surface receptors for the recognition of pathogen invasion and the inflammatory environment. Those include G-protein-coupled chemokine and chemoattractant receptors, Fc-receptors, adhesion receptors such as selectins/selectin ligands and integrins, various cytokine receptors, as well as innate immune receptors such as Toll-like receptors and C-type lectins. The various cell surface receptors trigger very diverse signal transduction pathways including activation of heterotrimeric and monomeric G-proteins, receptor-induced and store-operated Ca(2+) signals, protein and lipid kinases, adapter proteins and cytoskeletal rearrangement. Here we provide an overview of the receptors involved in neutrophil activation and the intracellular signal transduction processes they trigger. This knowledge is crucial for understanding how neutrophils participate in antimicrobial host defense and inflammatory tissue damage and may also point to possible future targets of the pharmacological therapy of neutrophil-mediated autoimmune or inflammatory diseases.
Keywords: ADAP; Abelson leukemia proto-oncogene; Abl; Asc; B-cell receptor; BCR; C-type lectin; C3G; CALDAG-GEFI; CARD; CEACAM3; CHO; CLEC; Chinese hamster ovary cells; Crk SH3 domain-binding guanine nucleotide exchange factor (RapGEF1); DAG; DAP12; DISC; DNAX activating protein 12; E-selectin ligand 1; ERK; ERM; ESL-1; Epac1; FADD; Fas-associated protein with death domain; Fc-receptor; Fc-receptor γ-chain; FcR; FcRγ; Fgr; G protein-coupled receptor; G-CSF; GAP; GM-CSF; GPCR; GPCR kinase; GPI; GRK; GTPase activating protein; Gardner–Rasheed feline sarcoma proto-oncogene; Hck; ICAM-1; IFN; IFN regulatory factor; IKK; IL; IL-1 receptor-associated kinase; IP(3); IRAK; IRF; ITAM; Inflammation; IκB; IκB kinase; JAK; JNK; Janus kinase; Kinases; LAD; LFA-1; LTB(4); LTβ; MAP kinase; MAP kinase kinase; MAP kinase-associated protein kinase; MAPKAP-kinase; MDA5; MDL-1; MIP; MKK; Mac-1; Mcl; MyD88; NF-κB; NLRP3; NOD; NOD-like receptor family, pyrin domain containing 3; Neutrophils; OSCAR; P-selectin glycoprotein ligand; PAF; PAK; PI3K; PIP(3); PIR; PKB; PKC; PLC; PSGL-1; RANK; RIG; RIP3; ROS; Rac; Rap; Ras-related C3 botulinum toxin substrate; Ras-related protein; Receptors; Rous sarcoma virus proto-oncogene; SAP130; SH2; SH2 domain-containing leukocyte protein of 76kDa; SH2 domain-containing protein tyrosine phosphatase 1; SHP-1; SLP-76; SOCS; STAT; Signaling; Sin3A-associated protein of 130kDa; Src; Src-homology 2 domain; Syk; T-cell receptor; TAK; TCR; TGFβ; TGFβ-activated kinase 1; TLR; TNF; TNF receptor-associated factor; TNF-related apoptosis-inducing ligand; TNFR1-associated death domain protein; TRADD; TRAF; TRAIL; TREM; Toll-like receptor; Tyk2; VASP; VCAM-1; VLA-4; ZAP-70; adhesion and degranulation promoting adapter protein (Fyb, SLAP-130); apoptosis-associated speck-like protein containing a CARD; c-Jun N-terminal kinase; cIAP; calcium and DAG-regulated guanine nucleotide exchange factor I; carcinoembryonic antigen-related cell adhesion molecule 3 (CD66b); caspase activation and recruitment domain; cellular inhibitor of apoptosis; death-inducing signaling complex; diacyl-glycerol; exchange protein activated by cyclic AMP 1; extracellular signal-regulated kinase; ezrin-radixin-moesin; fMLP; formly-Met-Leu-Phe; glycosylphosphatidylinositol anchor; granulocyte colony-stimulating factor; granulocyte/monocyte colony-stimulating factor; hematopoietic cell kinase; immunoreceptor tyrosine-based activation motif; inhibitor of NF-κB; inositol-tris-phosphate; intercellular adhesion molecule 1; interferon; interleukin; leukocyte adhesion deficiency; leukotriene B(4); lymphocyte function-associated receptor 1 (α(L)β(2) integrin); lymphotoxin β; macrophage C-type lectin; macrophage antigen 1 (α(M)β(2) integrin); macrophage inflammatory protein; melanoma differentiation-associated protein 5; mitogen-activated protein kinase; myeloid DAP12-associating lectin 1; myeloid differentiation protein 88; nuclear factor κB; nucleotide-binding oligomerization domain containing protein; osteoclast-associated receptor; p21-activated kinase; paired immunoglobulin-like receptor; phoshoinositide-3-kinase; phosphatidylinositol-3-phosphate; phospholipase C; platelet activating factor; protein kinase B; protein kinase C; reactive oxygen species; receptor activator of NF-κB; receptor-interacting serine-threonine protein kinase 3; retinoic acid-inducible gene; signal transducer and activator of transcription; spleen tyrosine kinase; suppressor of cytokine signaling; transforming growth factor β; triggering receptor expressed on myeloid cells; tumor necrosis factor; tyrosine protein kinase 2; vascular cell adhesion molecule 1; vasodilator-stimulated phosphoprotein; very late antigen 4 (α(4)β(1) integrin); ζ-chain-associated protein of 70kDa.
© 2013. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. - PubMed
-
- Borregaard N., Cowland J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. - PubMed
-
- Migeotte I., Communi D., Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

