Green tea phenolics inhibit butyrate-induced differentiation of colon cancer cells by interacting with monocarboxylate transporter 1
- PMID: 23994611
- PMCID: PMC4889458
- DOI: 10.1016/j.bbadis.2013.08.009
Green tea phenolics inhibit butyrate-induced differentiation of colon cancer cells by interacting with monocarboxylate transporter 1
Abstract
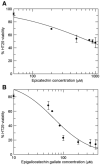
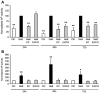
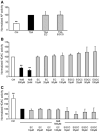
Diet has a significant impact on colorectal cancer and both dietary fiber and plant-derived compounds have been independently shown to be inversely related to colon cancer risk. Butyrate (NaB), one of the principal products of dietary fiber fermentation, induces differentiation of colon cancer cell lines by inhibiting histone deacetylases (HDACs). On the other hand, (-)-epicatechin (EC) and (-)-epigallocatechin gallate (EGCG), two abundant phenolic compounds of green tea, have been shown to exhibit antitumoral properties. In this study we used colon cancer cell lines to study the cellular and molecular events that take place during co-treatment with NaB, EC and EGCG. We found that (i) polyphenols EC and EGCG fail to induce differentiation of colon adenocarcinoma cell lines; (ii) polyphenols EC and EGCG reduce NaB-induced differentiation; (iii) the effect of the polyphenols is specific for NaB, since differentiation induced by other agents, such as trichostatin A (TSA), was unaltered upon EC and EGCG treatment, and (iv) is independent of the HDAC inhibitory activity of NaB. Also, (v) polyphenols partially reduce cellular NaB; and (vi) on a molecular level, reduction of cellular NaB uptake by polyphenols is achieved by impairing the capacity of NaB to relocalize its own transporter (monocarboxylate transporter 1, MCT1) in the plasma membrane. Our findings suggest that beneficial effects of NaB on colorectal cancer may be reduced by green tea phenolic supplementation. This valuable information should be of assistance in choosing a rational design for more effective diet-driven therapeutic interventions in the prevention or treatment of colorectal cancer.
Keywords: (−)-epicatechin; (−)-epigallocatechin gallate; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; AP; Butyrate; Colon cancer; Differentiation; EC; EGCG; Epicatechin; Epigallocatechin gallate; HDAC; MCT1; MTT; NaB; TSA; alkaline phosphatase; butyrate; histone deacetylase; monocarboxylate transporter 1; trichostatin A.
© 2013.
Conflict of interest statement
Figures
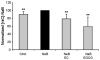
Similar articles
-
Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate.Exp Cell Res. 2014 May 15;324(1):40-53. doi: 10.1016/j.yexcr.2014.01.024. Epub 2014 Feb 8. Exp Cell Res. 2014. PMID: 24518414 Free PMC article.
-
Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols.Carcinogenesis. 1998 Apr;19(4):611-6. doi: 10.1093/carcin/19.4.611. Carcinogenesis. 1998. PMID: 9600345
-
Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea.Nutrients. 2012 Nov 8;4(11):1679-91. doi: 10.3390/nu4111679. Nutrients. 2012. PMID: 23201840 Free PMC article.
-
New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate.Redox Biol. 2014 Jan 10;2:187-95. doi: 10.1016/j.redox.2013.12.022. eCollection 2014. Redox Biol. 2014. PMID: 24494192 Free PMC article. Review.
-
Green tea polyphenols: biology and therapeutic implications in cancer.Front Biosci. 2007 Sep 1;12:4881-99. doi: 10.2741/2435. Front Biosci. 2007. PMID: 17569617 Review.
Cited by
-
Green Tea Catechins Induce Inhibition of PTP1B Phosphatase in Breast Cancer Cells with Potent Anti-Cancer Properties: In Vitro Assay, Molecular Docking, and Dynamics Studies.Antioxidants (Basel). 2020 Nov 30;9(12):1208. doi: 10.3390/antiox9121208. Antioxidants (Basel). 2020. PMID: 33266280 Free PMC article.
-
Research Trends and Hotspots Analysis Related to Monocarboxylate Transporter 1: A Study Based on Bibliometric Analysis.Int J Environ Res Public Health. 2019 Mar 27;16(7):1091. doi: 10.3390/ijerph16071091. Int J Environ Res Public Health. 2019. PMID: 30934693 Free PMC article.
-
Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer.World J Gastroenterol. 2020 Feb 14;26(6):562-597. doi: 10.3748/wjg.v26.i6.562. World J Gastroenterol. 2020. PMID: 32103869 Free PMC article. Review.
-
Regulation of colonic epithelial butyrate transport: Focus on colorectal cancer.Porto Biomed J. 2016 Jul-Aug;1(3):83-91. doi: 10.1016/j.pbj.2016.04.004. Epub 2016 Jul 1. Porto Biomed J. 2016. PMID: 32258556 Free PMC article. Review.
-
Phytochemicals Target Multiple Metabolic Pathways in Cancer.Antioxidants (Basel). 2023 Nov 17;12(11):2012. doi: 10.3390/antiox12112012. Antioxidants (Basel). 2023. PMID: 38001865 Free PMC article. Review.
References
-
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. - PubMed
-
- Watson AJ, Collins PD. Colon cancer: a civilization disorder. Digest Dis (Basel, Switzerland) 2011;29:222–228. - PubMed
-
- Ferguson LR, Chavan RR, Harris PJ. Changing concepts of dietary fiber: implications for carcinogenesis. Nutr Cancer. 2001;39:155–169. - PubMed
-
- Juskiewicz J, Milala J, Jurgonski A, Krol B, Zdunczyk Z. Nutrition. Vol. 27. Burbank Los Angeles County; Calif: 2011. Consumption of polyphenol concentrate with dietary fructo-oligosaccharides enhances cecal metabolism of quercetin glycosides in rats; pp. 351–357. - PubMed
-
- Kosmala M, Kolodziejczyk K, Zdunczyk Z, Juskiewicz J, Boros D. Chemical composition of natural and polyphenol-free apple pomace and the effect of this dietary ingredient on intestinal fermentation and serum lipid parameters in rats. J Agric Food Chem. 2011;59:9177–9185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

