The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity
- PMID: 23994691
- PMCID: PMC3938998
- DOI: 10.1016/j.mam.2013.08.002
The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity
Abstract
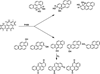
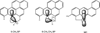
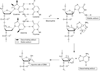
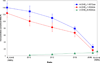
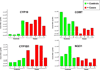
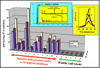
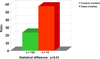
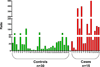
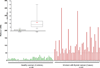
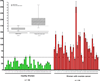
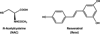
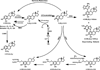
Elucidation of estrogen carcinogenesis required a few fundamental discoveries made by studying the mechanism of carcinogenesis of polycyclic aromatic hydrocarbons (PAH). The two major mechanisms of metabolic activation of PAH involve formation of radical cations and diol epoxides as ultimate carcinogenic metabolites. These intermediates react with DNA to yield two types of adducts: stable adducts that remain in DNA unless removed by repair and depurinating adducts that are lost from DNA by cleavage of the glycosyl bond between the purine base and deoxyribose. The potent carcinogenic PAH benzo[a]pyrene, dibenzo[a,l]pyrene, 7,12-dimethylbenz[a]anthracene and 3-methylcholanthrene predominantly form depurinating DNA adducts, leaving apurinic sites in the DNA that generate cancer-initiating mutations. This was discovered by correlation between the depurinating adducts formed in mouse skin by treatment with benzo[a]pyrene, dibenzo[a,l]pyrene or 7,12-dimethylbenz[a]anthracene and the site of mutations in the Harvey-ras oncogene in mouse skin papillomas initiated by one of these PAH. By applying some of these fundamental discoveries in PAH studies to estrogen carcinogenesis, the natural estrogens estrone (E1) and estradiol (E2) were found to be mutagenic and carcinogenic through formation of the depurinating estrogen-DNA adducts 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua. These adducts are generated by reaction of catechol estrogen quinones with DNA, analogously to the DNA adducts obtained from the catechol quinones of benzene, naphthalene, and the synthetic estrogens diethylstilbestrol and hexestrol. This is a weak mechanism of cancer initiation. Normally, estrogen metabolism is balanced and few estrogen-DNA adducts are formed. When estrogen metabolism becomes unbalanced, more catechol estrogen quinones are generated, resulting in higher levels of estrogen-DNA adducts, which can be used as biomarkers of unbalanced estrogen metabolism and, thus, cancer risk. The ratio of estrogen-DNA adducts to estrogen metabolites and conjugates has repeatedly been found to be significantly higher in women at high risk for breast cancer, compared to women at normal risk. These results indicate that formation of estrogen-DNA adducts is a critical factor in the etiology of breast cancer. Significantly higher adduct ratios have been observed in women with breast, thyroid or ovarian cancer. In the women with ovarian cancer, single nucleotide polymorphisms in the genes for two enzymes involved in estrogen metabolism indicate risk for ovarian cancer. When polymorphisms produce high activity cytochrome P450 1B1, an activating enzyme, and low activity catechol-O-methyltransferase, a protective enzyme, in the same woman, she is almost six times more likely to have ovarian cancer. These results indicate that formation of estrogen-DNA adducts is a critical factor in the etiology of ovarian cancer. Significantly higher ratios of estrogen-DNA adducts to estrogen metabolites and conjugates have also been observed in men with prostate cancer or non-Hodgkin lymphoma, compared to healthy men without cancer. These results also support a critical role of estrogen-DNA adducts in the initiation of cancer. Starting from the perspective that unbalanced estrogen metabolism can lead to increased formation of catechol estrogen quinones, their reaction with DNA to form adducts, and generation of cancer-initiating mutations, inhibition of estrogen-DNA adduct formation would be an effective approach to preventing a variety of human cancers. The dietary supplements resveratrol and N-acetylcysteine can act as preventing cancer agents by keeping estrogen metabolism balanced. These two compounds can reduce the formation of catechol estrogen quinones and/or their reaction with DNA. Therefore, resveratrol and N-acetylcysteine provide a widely applicable, inexpensive approach to preventing many of the prevalent types of human cancer.
Keywords: 1,4-Michael addition mechanism; Apurinic sites in DNA; Cancer prevention by N-acetylcysteine and resveratrol; Carcinogenicity of 4-hydroxyestrogens; Cytochrome P4501B1; Depurinating PAH–DNA adducts; Depurinating estrogen–DNA adducts; Estrogen-3,4-quinones; Genotoxicity of estrogens; Hormonal carcinogenesis; Imbalance of estrogen homeostasis; Mutations by error-prone repair; Nonhormonal carcinogenesis; PAH diol epoxides; PAH radical cations; Preneoplastic mutations.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




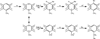








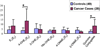








References
-
- Alpert AJ, Cavalieri EL. Metabolism of 6-substituted benzo[a]pyrene derivatives:O-Dealkylation and regiospecificity in aromatic hydroxylations. J. Med. Chem. 1980;23:919–927. - PubMed
-
- Amin S, Huie K, Balanikas G, Hecht SS, Pataki J, Harvey RG. High stereoselectivity in mouse skin metabolic activation of methylchrysenes to tumorigenic dihydrodiols. Cancer Res. 1987;47:3613–3617. - PubMed
-
- Amin S, Huie K, Melikian AA, Leszczynska JM, Hecht SS. Comparative metabolic activation in mouse skin of the weak carcinogen 6-methylchrysene and the strong carcinogen 5-methylchrysene. Cancer Res. 1985;45:6406–6442. - PubMed
-
- Arcos JC, Argus MF. Chemical induction of cancer. VolIIA. New York: Academic Press; 1974.
-
- Atkinson JK, Hollenberg PF, Ingold KU, Johnson CC, Le Tadic MH, Newcomb M, Putt DA. Cytochrome P450-catalyzed hydroxylation of hydrocarbons: Kinetic deuterium isotope effects for the hydroxylation of an ultrafast radical clock. Biochemistry. 1994;33:10630–10637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

