Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition
- PMID: 23995071
- PMCID: PMC6379206
- DOI: 10.1038/nn.3509
Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition
Abstract
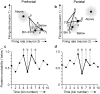
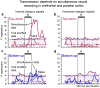
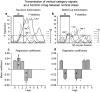
Prefrontal cortex influences behavior largely through its connections with other association cortices; however, the nature of the information conveyed by prefrontal output signals and what effect these signals have on computations performed by target structures is largely unknown. To address these questions, we simultaneously recorded the activity of neurons in prefrontal and posterior parietal cortices of monkeys performing a rule-based spatial categorization task. Parietal cortex receives direct prefrontal input, and parietal neurons, like their prefrontal counterparts, exhibit signals that reflect rule-based cognitive processing in this task. By analyzing rapid fluctuations in the cognitive information encoded by activity in the two areas, we obtained evidence that signals reflecting rule-dependent categories were selectively transmitted in a top-down direction from prefrontal to parietal neurons, suggesting that prefrontal output is important for the executive control of distributed cognitive processing.
Figures


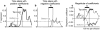

Comment in
-
Slicing a pie is no piece of cake.Nat Neurosci. 2013 Oct;16(10):1364-6. doi: 10.1038/nn.3520. Nat Neurosci. 2013. PMID: 24067288 No abstract available.
References
-
- Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci. 2006;23:161–179. - PubMed
-
- Schwartz ML, Goldman-Rakic PS. Callosal and intrahemispheric connectivity of the prefrontal association cortex in rhesus monkey: relation between intraparietal and principal sulcal cortex. J Comp Neurol. 1984;226:403–420. - PubMed
-
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. - PubMed
-
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. - PubMed
-
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Mountcastle VB, Plum F, Geiger SR, editors. Handbook of Physiology The Nervous System Higher Functions of the Brain. Am. Physiol. Soc.; Bethesda, MD: 1987. pp. 373–417.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

