Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity
- PMID: 23995283
- PMCID: PMC4079117
- DOI: 10.1038/nm.3321
Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity
Abstract
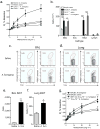
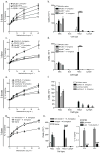
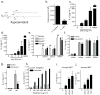
Aspergillus fumigatus is a saprophytic fungus that is ubiquitous in the environment and is commonly associated with allergic sensitization and severe asthma in humans. Although A. fumigatus is recognized by multiple microbial pattern-recognition receptors, we found that an A. fumigatus-derived glycosphingolipid, asperamide B, directly activates invariant natural killer T (iNKT) cells in vitro in a CD1d-restricted, MyD88-independent and dectin-1-independent fashion. Moreover, asperamide B, when loaded onto CD1d, directly stained, and was sufficient to activate, human and mouse iNKT cells. In vivo, asperamide B rapidly induced airway hyperreactivity, which is a cardinal feature of asthma, by activating pulmonary iNKT cells in an interleukin-33 (IL-33)-ST2-dependent fashion. Asperamide B is thus the first fungal glycolipid found to directly activate iNKT cells. These results extend the range of microorganisms that can be directly detected by iNKT cells to the kingdom of fungi and may explain how A. fumigatus can induce severe chronic respiratory diseases in humans.
Figures
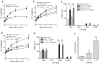

Comment in
-
NKT cells: the smoking gun in fungal-induced asthma?Nat Med. 2013 Oct;19(10):1210-1. doi: 10.1038/nm.3360. Nat Med. 2013. PMID: 24100979
References
-
- O'Connor G T, et al. Airborne fungi in the homes of children with asthma in low-income urban communities: The Inner-City Asthma Study. J Allergy Clin Immunol. 2004;114:599–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

