Human amniotic fluid stem cell differentiation along smooth muscle lineage
- PMID: 23995291
- PMCID: PMC6188351
- DOI: 10.1096/fj.12-218578
Human amniotic fluid stem cell differentiation along smooth muscle lineage
Abstract
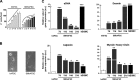
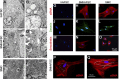
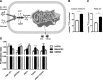
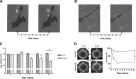
Functional smooth muscle engineering requires isolation and expansion of smooth muscle cells (SMCs), and this process is particularly challenging for visceral smooth muscle tissue where progenitor cells have not been clearly identified. Herein we showed for the first time that efficient SMCs can be obtained from human amniotic fluid stem cells (hAFSCs). Clonal lines were generated from c-kit(+) hAFSCs. Differentiation toward SM lineage (SMhAFSCs) was obtained using a medium conditioned by PDGF-BB and TGF-β1. Molecular assays revealed higher level of α smooth muscle actin (α-SMA), desmin, calponin, and smoothelin in SMhAFSCs when compared to hAFSCs. Ultrastructural analysis demonstrated that SMhAFSCs also presented in the cytoplasm increased intermediate filaments, dense bodies, and glycogen deposits like SMCs. SMhAFSC metabolism evaluated via mass spectrometry showed higher glucose oxidation and an enhanced response to mitogenic stimuli in comparison to hAFSCs. Patch clamp of transduced hAFSCs with lentiviral vectors encoding ZsGreen under the control of the α-SMA promoter was performed demonstrating that SMhAFSCs retained a smooth muscle cell-like electrophysiological fingerprint. Eventually SMhAFSCs contractility was evident both at single cell level and on a collagen gel. In conclusion, we showed here that hAFSCs under selective culture conditions are able to give rise to functional SMCs.
Keywords: fetal cells; multipotent; myogenic; regenerative medicine; tissue engineering.
Conflict of interest statement
The authors thank Dr. Bertrand Vernay for assistance with time lapse microscopy, Ayad Eddaoudi for his valuable help in cell sorting, Dr. Sara Paccosi and Dr. Claudia Musilli for their support with cell cultures, and Dr. Pedro Lei and Dr. Waseem Qasim for their support with transduction.
Figures

Similar articles
-
Differentiated markers in undifferentiated cells: expression of smooth muscle contractile proteins in multipotent bone marrow mesenchymal stem cells.Dev Growth Differ. 2013 Jun;55(5):591-605. doi: 10.1111/dgd.12052. Epub 2013 Apr 4. Dev Growth Differ. 2013. PMID: 23557080
-
The proliferation and differentiation of placental-derived multipotent cells into smooth muscle cells on fibrillar collagen.Biomaterials. 2010 May;31(15):4367-75. doi: 10.1016/j.biomaterials.2010.02.011. Epub 2010 Mar 3. Biomaterials. 2010. PMID: 20199810
-
Smooth Muscle-Like Cells Generated from Human Mesenchymal Stromal Cells Display Marker Gene Expression and Electrophysiological Competence Comparable to Bladder Smooth Muscle Cells.PLoS One. 2015 Dec 16;10(12):e0145153. doi: 10.1371/journal.pone.0145153. eCollection 2015. PLoS One. 2015. PMID: 26673782 Free PMC article.
-
Amniotic fluid stem cells as a novel strategy for the treatment of fetal and neonatal neurological diseases.Placenta. 2021 Jan 15;104:247-252. doi: 10.1016/j.placenta.2021.01.009. Epub 2021 Jan 12. Placenta. 2021. PMID: 33461069 Review.
-
The potential use of stem cells derived from human amniotic fluid in renal diseases.Kidney Int Suppl (2011). 2011 Sep;1(3):77-82. doi: 10.1038/kisup.2011.18. Kidney Int Suppl (2011). 2011. PMID: 25028628 Free PMC article. Review.
Cited by
-
Building gut from scratch - progress and update of intestinal tissue engineering.Nat Rev Gastroenterol Hepatol. 2022 Jul;19(7):417-431. doi: 10.1038/s41575-022-00586-x. Epub 2022 Mar 3. Nat Rev Gastroenterol Hepatol. 2022. PMID: 35241800 Review.
-
Glycoprotein M6B Interacts with TβRI to Activate TGF-β-Smad2/3 Signaling and Promote Smooth Muscle Cell Differentiation.Stem Cells. 2019 Feb;37(2):190-201. doi: 10.1002/stem.2938. Epub 2018 Nov 27. Stem Cells. 2019. PMID: 30372567 Free PMC article.
-
An Engineered Living Intestinal Muscle Patch Produces Macroscopic Contractions that can Mix and Break Down Artificial Intestinal Contents.Adv Mater. 2023 Apr;35(15):e2207255. doi: 10.1002/adma.202207255. Epub 2023 Mar 4. Adv Mater. 2023. PMID: 36779454 Free PMC article.
-
Tissue engineering of urinary bladder and urethra: advances from bench to patients.ScientificWorldJournal. 2013 Dec 24;2013:154564. doi: 10.1155/2013/154564. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24453796 Free PMC article. Review.
-
Cardiac Restoration Stemming From the Placenta Tree: Insights From Fetal and Perinatal Cell Biology.Front Physiol. 2018 Apr 11;9:385. doi: 10.3389/fphys.2018.00385. eCollection 2018. Front Physiol. 2018. PMID: 29695981 Free PMC article. Review.
References
-
- Nakase Y., Nakamura T., Kin S., Nakashima S., Yoshikawa T., Kuriu Y., Sakakura C., Yamagishi H., Hamuro J., Ikada Y., Otsuji E., Hagiwara A. (2008) Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J. Thorac. Cardiovasc. Surg. , 850–859 - PubMed
-
- Sala F. G., Kunisaki S. M., Ochoa E. R., Vacanti J., Grikscheit T. C. (2009) Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J. Surg. Res. , 205–212 - PubMed
-
- Atala A., Bauer S. B., Soker S., Yoo J. J., Retik A. B. (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet , 1241–1246 - PubMed
-
- Badylak S. F., Weiss D. J., Caplan A., Macchiarini P. (2012) Engineered whole organs and complex tissues. Lancet , 943–952 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

