Regulation of host hemoglobin binding by the Staphylococcus aureus Clp proteolytic system
- PMID: 23995637
- PMCID: PMC3811588
- DOI: 10.1128/JB.00505-13
Regulation of host hemoglobin binding by the Staphylococcus aureus Clp proteolytic system
Abstract
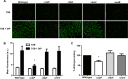
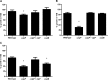
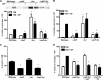
Protein turnover is a key process for bacterial survival mediated by intracellular proteases. Proteolytic degradation reduces the levels of unfolded and misfolded peptides that accumulate in the cell during stress conditions. Three intracellular proteases, ClpP, HslV, and FtsH, have been identified in the Gram-positive bacterium Staphylococcus aureus, a pathogen responsible for significant morbidity and mortality worldwide. Consistent with their crucial role in protein turnover, ClpP, HslV, and FtsH affect a number of cellular processes, including metabolism, stress responses, and virulence. The ClpP protease is believed to be the principal degradation machinery in S. aureus. This study sought to identify the effect of the Clp protease on the iron-regulated surface determinant (Isd) system, which extracts heme-iron from host hemoglobin during infection and is critical to S. aureus pathogenesis. Inactivation of components of the Clp protease alters abundance of several Isd proteins, including the hemoglobin receptor IsdB. Furthermore, the observed changes in IsdB abundance are the result of transcriptional regulation, since transcription of isdB is decreased by clpP or clpX inactivation. In contrast, inactivation of clpC enhances isdB transcription and protein abundance. Loss of clpP or clpX impairs host hemoglobin binding and utilization and results in severe virulence defects in a systemic mouse model of infection. These findings suggest that the Clp proteolytic system is important for regulating nutrient iron acquisition in S. aureus. The Clp protease and Isd complex are widely conserved in bacteria; therefore, these data reveal a novel Clp-dependent regulation pathway that may be present in other bacterial pathogens.
Figures





References
-
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 - PubMed
-
- Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG, International Collaboration on Endocarditis Merged Database Group 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 41:507–514 - PubMed
-
- Purcell K, Fergie J. 2005. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children's Hospital. Arch. Pediatr. Adolesc. Med. 159:980–985 - PubMed
-
- Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

