Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2
- PMID: 23995678
- PMCID: PMC3791160
- DOI: 10.1038/nature12501
Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2
Abstract
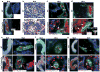
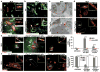
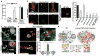
Circulating lymphocytes continuously enter lymph nodes for immune surveillance through specialized blood vessels named high endothelial venules, a process that increases markedly during immune responses. How high endothelial venules (HEVs) permit lymphocyte transmigration while maintaining vascular integrity is unknown. Here we report a role for the transmembrane O-glycoprotein podoplanin (PDPN, also known as gp38 and T1α) in maintaining HEV barrier function. Mice with postnatal deletion of Pdpn lost HEV integrity and exhibited spontaneous bleeding in mucosal lymph nodes, and bleeding in the draining peripheral lymph nodes after immunization. Blocking lymphocyte homing rescued bleeding, indicating that PDPN is required to protect the barrier function of HEVs during lymphocyte trafficking. Further analyses demonstrated that PDPN expressed on fibroblastic reticular cells, which surround HEVs, functions as an activating ligand for platelet C-type lectin-like receptor 2 (CLEC-2, also known as CLEC1B). Mice lacking fibroblastic reticular cell PDPN or platelet CLEC-2 exhibited significantly reduced levels of VE-cadherin (also known as CDH5), which is essential for overall vascular integrity, on HEVs. Infusion of wild-type platelets restored HEV integrity in Clec-2-deficient mice. Activation of CLEC-2 induced release of sphingosine-1-phosphate from platelets, which promoted expression of VE-cadherin on HEVs ex vivo. Furthermore, draining peripheral lymph nodes of immunized mice lacking sphingosine-1-phosphate had impaired HEV integrity similar to Pdpn- and Clec-2-deficient mice. These data demonstrate that local sphingosine-1-phosphate release after PDPN-CLEC-2-mediated platelet activation is critical for HEV integrity during immune responses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
-
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nature Immunol. 2006;7:344–353. - PubMed
-
- Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nature Rev Immunol. 2012;12:762–773. - PubMed
-
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. - PubMed
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nature Rev Immunol. 2003;3:867–878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM103441/GM/NIGMS NIH HHS/United States
- HL093242/HL/NHLBI NIH HHS/United States
- P20 GM103441/GM/NIGMS NIH HHS/United States
- HL065590/HL/NHLBI NIH HHS/United States
- HL112788/HL/NHLBI NIH HHS/United States
- I01 BX001984/BX/BLRD VA/United States
- R01 GM097747/GM/NIGMS NIH HHS/United States
- R01 HL112788/HL/NHLBI NIH HHS/United States
- S10 RR024598/RR/NCRR NIH HHS/United States
- HL103432/HL/NHLBI NIH HHS/United States
- R01 HL065590/HL/NHLBI NIH HHS/United States
- P20 GM103527/GM/NIGMS NIH HHS/United States
- P20 RR018758/RR/NCRR NIH HHS/United States
- R01 HL093242/HL/NHLBI NIH HHS/United States
- R01 HL103432/HL/NHLBI NIH HHS/United States
- HL085607/HL/NHLBI NIH HHS/United States
- P01 HL085607/HL/NHLBI NIH HHS/United States
- GM097747/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

