Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens
- PMID: 23995682
- PMCID: PMC3825626
- DOI: 10.1038/nature12503
Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens
Abstract
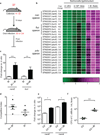
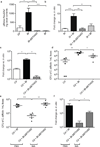
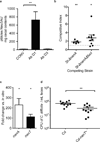
The human intestine, colonized by a dense community of resident microbes, is a frequent target of bacterial pathogens. Undisturbed, this intestinal microbiota provides protection from bacterial infections. Conversely, disruption of the microbiota with oral antibiotics often precedes the emergence of several enteric pathogens. How pathogens capitalize upon the failure of microbiota-afforded protection is largely unknown. Here we show that two antibiotic-associated pathogens, Salmonella enterica serovar Typhimurium (S. typhimurium) and Clostridium difficile, use a common strategy of catabolizing microbiota-liberated mucosal carbohydrates during their expansion within the gut. S. typhimurium accesses fucose and sialic acid within the lumen of the gut in a microbiota-dependent manner, and genetic ablation of the respective catabolic pathways reduces its competitiveness in vivo. Similarly, C. difficile expansion is aided by microbiota-induced elevation of sialic acid levels in vivo. Colonization of gnotobiotic mice with a sialidase-deficient mutant of Bacteroides thetaiotaomicron, a model gut symbiont, reduces free sialic acid levels resulting in C. difficile downregulating its sialic acid catabolic pathway and exhibiting impaired expansion. These effects are reversed by exogenous dietary administration of free sialic acid. Furthermore, antibiotic treatment of conventional mice induces a spike in free sialic acid and mutants of both Salmonella and C. difficile that are unable to catabolize sialic acid exhibit impaired expansion. These data show that antibiotic-induced disruption of the resident microbiota and subsequent alteration in mucosal carbohydrate availability are exploited by these two distantly related enteric pathogens in a similar manner. This insight suggests new therapeutic approaches for preventing diseases caused by antibiotic-associated pathogens.
Figures
Comment in
-
Finding a sugary foothold: how antibiotics pave the way for enteric pathogens.Cell Host Microbe. 2013 Sep 11;14(3):225-7. doi: 10.1016/j.chom.2013.08.008. Cell Host Microbe. 2013. PMID: 24034607
-
An antibiotic-altered microbiota provides fuel for the enteric foe.Cell Res. 2014 Jan;24(1):5-6. doi: 10.1038/cr.2013.142. Epub 2013 Oct 29. Cell Res. 2014. PMID: 24165893 Free PMC article.
-
How enteric pathogens know they hit the sweet spot.Future Microbiol. 2014;9(1):13-6. doi: 10.2217/fmb.13.141. Future Microbiol. 2014. PMID: 24328376 Free PMC article.
References
-
- Doorduyn Y, Van Den Brandhof WE, Van Duynhoven YT, Wannet WJ, Van Pelt W. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiology and infection. 2006;134:617–626. - PMC - PubMed
-
- Pavia AT, et al. Epidemiologic evidence that prior antimicrobial exposure decreases resistance to infection by antimicrobial-sensitive Salmonella. The Journal of infectious diseases. 1990;161:255–260. - PubMed
-
- Pepin J, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–1260. - PubMed
-
- Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science (New York, N.Y. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

