Structural insight into the biogenesis of β-barrel membrane proteins
- PMID: 23995689
- PMCID: PMC3779476
- DOI: 10.1038/nature12521
Structural insight into the biogenesis of β-barrel membrane proteins
Abstract
β-barrel membrane proteins are essential for nutrient import, signalling, motility and survival. In Gram-negative bacteria, the β-barrel assembly machinery (BAM) complex is responsible for the biogenesis of β-barrel membrane proteins, with homologous complexes found in mitochondria and chloroplasts. Here we describe the structure of BamA, the central and essential component of the BAM complex, from two species of bacteria: Neisseria gonorrhoeae and Haemophilus ducreyi. BamA consists of a large periplasmic domain attached to a 16-strand transmembrane β-barrel domain. Three structural features shed light on the mechanism by which BamA catalyses β-barrel assembly. First, the interior cavity is accessible in one BamA structure and conformationally closed in the other. Second, an exterior rim of the β-barrel has a distinctly narrowed hydrophobic surface, locally destabilizing the outer membrane. And third, the β-barrel can undergo lateral opening, suggesting a route from the interior cavity in BamA into the outer membrane.
Figures

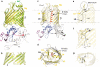

Comment in
-
Lateral gates: β-barrels get in on the act.Nat Struct Mol Biol. 2013 Nov;20(11):1237-9. doi: 10.1038/nsmb.2709. Nat Struct Mol Biol. 2013. PMID: 24197163 No abstract available.
References
-
- Dalbey RE, Wang P, Kuhn A. Assembly of bacterial inner membrane proteins. Annu Rev Biochem. 2011;80:161–187. - PubMed
-
- White SH, von Heijne G. How translocons select transmembrane helices. Annu Rev Biophys. 2008;37:23–42. - PubMed
-
- du Plessis DJ, Nouwen N, Driessen AJ. The Sec translocase. Biochim Biophys Acta. 2011;1808:851–865. - PubMed
-
- Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol. 2005;21:529–550. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

