Slug expression inhibits calcitriol-mediated sensitivity to radiation in colorectal cancer
- PMID: 23996472
- PMCID: PMC3858479
- DOI: 10.1002/mc.22054
Slug expression inhibits calcitriol-mediated sensitivity to radiation in colorectal cancer
Abstract
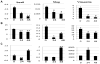
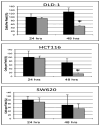
Recently, a reciprocal relationship between calcitriol and epithelial-to-mesenchymal transition has been described. Therefore, we hypothesized that calcitriol (1α,25-dihydroxyvitamin D₃) would enhance radiation sensitivity in colorectal cancer regulated by epithelial mesenchymal transition. Vitamin-D receptor, E-cadherin and vimentin protein as well as E-cadherin, Snail and Slug mRNA levels were assessed in a panel of human colorectal cancer cell lines at baseline and in response calcitriol. We defined cell lines as calcitriol sensitive based on demonstrating an enhanced epithelial phenotype with increased E-cadherin, reduced vimentin and decreased expression of Snail and Slug as well as decreased cellular migration in response to calcitriol. In calcitriol sensitive cells, including DLD-1 and HCT116, 24 h calcitriol pre-treatment enhanced the radiation sensitivity by 2.3- and 2.6-fold, respectively, at 4 Gy (P < 0.05). In contrast, SW620 cells with high baseline mesenchymal features including high Slug and vimentin expression with low E-cadherin expression demonstrated no significant radiation sensitizing response to calcitriol treatment. Similarly, transfection of Slug in the calcitriol sensitive colon cancer cell lines, DLD-1 and HCT 116, completely inhibited the radiation sensitizing effect of calcitriol. Collectively, we demonstrate that calcitriol can enhance the therapeutic effects of radiation in colon cancer cells and Slug expression mitigates this observed effect potentially representing an effective biomarker for calcitriol therapy.
Keywords: EMT; Slug; cancer; colorectal; radiation; vitamin D.
© 2013 Wiley Periodicals, Inc.
Figures





References
-
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. - PubMed
-
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

