Pituitary adenylate cyclase-activating polypeptide (PACAP) inhibits the slow afterhyperpolarizing current sIAHP in CA1 pyramidal neurons by activating multiple signaling pathways
- PMID: 23996525
- PMCID: PMC3920641
- DOI: 10.1002/hipo.22201
Pituitary adenylate cyclase-activating polypeptide (PACAP) inhibits the slow afterhyperpolarizing current sIAHP in CA1 pyramidal neurons by activating multiple signaling pathways
Abstract
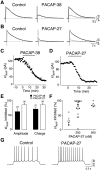
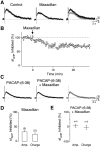
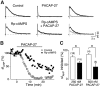
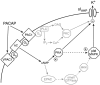
The slow afterhyperpolarizing current (sIAHP ) is a calcium-dependent potassium current that underlies the late phase of spike frequency adaptation in hippocampal and neocortical neurons. sIAHP is a well-known target of modulation by several neurotransmitters acting via the cyclic AMP (cAMP) and protein kinase A (PKA)-dependent pathway. The neuropeptide pituitary adenylate cyclase activating peptide (PACAP) and its receptors are present in the hippocampal formation. In this study we have investigated the effect of PACAP on the sIAHP and the signal transduction pathway used to modulate intrinsic excitability of hippocampal pyramidal neurons. We show that PACAP inhibits the sIAHP , resulting in a decrease of spike frequency adaptation, in rat CA1 pyramidal cells. The suppression of sIAHP by PACAP is mediated by PAC1 and VPAC1 receptors. Inhibition of PKA reduced the effect of PACAP on sIAHP, suggesting that PACAP exerts part of its inhibitory effect on sIAHP by increasing cAMP and activating PKA. The suppression of sIAHP by PACAP was also strongly hindered by the inhibition of p38 MAP kinase (p38 MAPK). Concomitant inhibition of PKA and p38 MAPK indicates that these two kinases act in a sequential manner in the same pathway leading to the suppression of sIAHP. Conversely, protein kinase C is not part of the signal transduction pathway used by PACAP to inhibit sIAHP in CA1 neurons. Our results show that PACAP enhances the excitability of CA1 pyramidal neurons by inhibiting the sIAHP through the activation of multiple signaling pathways, most prominently cAMP/PKA and p38 MAPK. Our findings disclose a novel modulatory action of p38 MAPK on intrinsic excitability and the sIAHP, underscoring the role of this current as a neuromodulatory hub regulated by multiple protein kinases in cortical neurons.
Keywords: hippocampus; neuropeptide; p38 MAP kinase; protein kinase A; slow afterhyperpolarization.
© 2013 The Authors. Hippocampus Published by Wiley Periodicals, Inc.
Figures




References
-
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. - PubMed
-
- Alger BE, Nicoll RA. Epileptiform burst afterhyperolarization: Calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980;210:1122–1124. - PubMed
-
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
-
- Borde M, Bonansco C, Buno W. The activity-dependent potentiation of the slow Ca2+-activated K+ current regulates synaptic efficacy in rat CA1 pyramidal neurons. Pflugers Arch. 1999;437:261–266. - PubMed
-
- Botelho LH, Rothermel JD, Coombs RV, Jastorff B. cAMP analog antagonists of cAMP action. Methods Enzymol. 1988;159:159–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

