Consensus paper: the cerebellum's role in movement and cognition
- PMID: 23996631
- PMCID: PMC4089997
- DOI: 10.1007/s12311-013-0511-x
Consensus paper: the cerebellum's role in movement and cognition
Abstract
While the cerebellum's role in motor function is well recognized, the nature of its concurrent role in cognitive function remains considerably less clear. The current consensus paper gathers diverse views on a variety of important roles played by the cerebellum across a range of cognitive and emotional functions. This paper considers the cerebellum in relation to neurocognitive development, language function, working memory, executive function, and the development of cerebellar internal control models and reflects upon some of the ways in which better understanding the cerebellum's status as a "supervised learning machine" can enrich our ability to understand human function and adaptation. As all contributors agree that the cerebellum plays a role in cognition, there is also an agreement that this conclusion remains highly inferential. Many conclusions about the role of the cerebellum in cognition originate from applying known information about cerebellar contributions to the coordination and quality of movement. These inferences are based on the uniformity of the cerebellum's compositional infrastructure and its apparent modular organization. There is considerable support for this view, based upon observations of patients with pathology within the cerebellum.
Figures

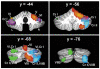
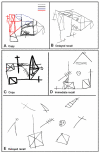

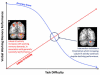



References
-
- Schmahmann JD. The cerebellum and cognition. Academic; London: 1997.
-
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71(1):44–56. - PubMed
-
- Baillieux H, Smet HJ, Paquier PF, De Deyn PP, Marien P. Cerebellar neurocognition: Insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110(8):763–73. - PubMed
-
- Kuper M, Dimitrova A, Thurling M, Maderwald S, Roths J, Elles HG, et al. Evidence for a motor and a non-motor domain in the human dentate nucleus—An fMRI study. Neuroimage. 2011;54(4):2612–22. - PubMed
-
- Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De LM, Leggio MG. Cerebellum and detection of sequences, from perception to cognition. Cerebellum. 2008;7(4):611–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

