Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease
- PMID: 23996934
- PMCID: PMC3799579
- DOI: 10.1002/emmm.201201584
Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease
Abstract
Identification of tissue-specific renal stem/progenitor cells with nephrogenic potential is a critical step in developing cell-based therapies for renal disease. In the human kidney, stem/progenitor cells are induced into the nephrogenic pathway to form nephrons until the 34 week of gestation, and no equivalent cell types can be traced in the adult kidney. Human nephron progenitor cells (hNPCs) have yet to be isolated. Here we show that growth of human foetal kidneys in serum-free defined conditions and prospective isolation of NCAM1(+) cells selects for nephron lineage that includes the SIX2-positive cap mesenchyme cells identifying a mitotically active population with in vitro clonogenic and stem/progenitor properties. After transplantation in the chick embryo, these cells-but not differentiated counterparts-efficiently formed various nephron tubule types. hNPCs engrafted and integrated in diseased murine kidneys and treatment of renal failure in the 5/6 nephrectomy kidney injury model had beneficial effects on renal function halting disease progression. These findings constitute the first definition of an intrinsic nephron precursor population, with major potential for cell-based therapeutic strategies and modelling of kidney disease.
Keywords: development; kidney stem cells; progenitor cells; regeneration; stem cells.
© 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures
Mouse embryonic kidney organ culture stained for Ncam1, Six2 and E-cad as indicated. An enlargement of the Ncam/Six2 signal is shown to emphasize the nuclear localization of Six2. White arrow illustrates the absence of Six2 signal in E-cad positive cells. An occurrence of Six2/E-cad positive cells is indicated with the asterisk. Images were obtained using Nikon A1R confocal microscope with and processed in ImageJ/Fiji software.
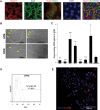
Morphology of hFK cells cultured in SFM or SCM after 3 days (passage 0 Day 3—left panels) and towards confluence (14 days in SFM or 7 days in SCM—right panels). Distinct borders appear in SFM cultures (arrows) whereas cells with different morphology (arrows) are observed in SCM culture. Cells were observed using a Nikon Digital Sight camera attached to a Nikon Eclipse TS100 microscope.
qRT-PCR analysis of gene expression in hFK cells cultured in SFM (three independent replicates). hPRT1 was used as endogenous control and SCM cells were used as the calibrator sample for RQ calculation (therefore = 1). Data were analysed using SDS 3.2 software.
Representative FACS analysis of NCAM1 expression in hFK cells cultured in SFM at passage1. Data is presented in a histogram graph showing NCAM1 staining in blue and the isotype controls staining (negative control) in red.
Immunofluorescence staining of NCAM1 (red) in total hFK cells cultured in SFM. Nuclei stained with Dapi (blue). Images were obtained using Olympus DP72 camera attached to Olympus BX51 fluorescence microscope and processed via cellSens standard software.
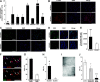
A. RT-PCR analysis of gene expression in NCAM1 cell fractions. GAPDH was used as endogenous control and NCAM1− cell were used as the calibrator sample for RQ calculation (therefore = 1). Data were analysed using SDS 3.2 software and presented as average RQ ± SDEV of three replicates. ***p < 0.001, *p < 0.05 versus NCAM1−.
B–D. Immunofluorescence staining of NCAM1+ and NCAM1− subpopulations for E-cadherin (E-cad) (B, red) vimentin (C, green) and SIX2 (D, green). Nuclei stained with Dapi (blue). (B–C) Images were obtained using Olympus DP72 camera attached to Olympus BX51 fluorescence microscope and processed via cellSens standard software, bar represents 200 μm. (D) Images were obtained using Zeiss LSM 510 confocal microscope, bar represents 50 μm.
E. Fluorescent quantification of SIX2 immunostaining as represented in (D).
F. Double labelling of sorted NCAM1+ cells for NCAM1 and SIX2: NCAM1 with DAPI (upper panel; red and blue channels), NCAM1 with SIX2 (lower panel; red and green channels), indicating both NCAM1+ SIX2+ (arrows) and NCAM1+ SIX2− (arrowheads) cells.
G. Graph represents percentage of NCAM1+ SIX2+ cells and NCAM1+ SIX2− cells.
H. Clonogenic efficiency of NCAM1+ cells sorted from hFK and cultured in SFM. Data are presented as average CE(%) ± SDEV. **p < 0.01 versus NCAM1−.
I. Representative morphology of NCAM1+ and NCAM1− clones. Cells were observed using a Nikon Digital Sight camera attached to a Nikon Eclipse TS100 microscope.
J. Clonogenic capacity of hFK cells treated with IMGN901(ADC 55 nM), huN901 (Ab55 nM) or not treated (control). Data are presented as average CE(%) ± SDEV. ***p < 0.001, *p < 0.05 versus control group.
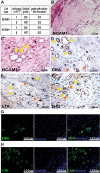
A. Summarizing table of chick CAM results (cell type, cells/egg, visible grafts and grafts with tubule-like formations).
B,C. H&E staining of paraffin-embedded sections of 5 × 105 NCAM1− (B) and 5 × 105 NCAM1+ (C) cells. Yellow arrows mark the tubule-like formations. The images were obtained using Scion colour digital camera attached to an Olympus BX51TF microscope.
D–F. Segment-specific IHC staining of DBA (D), LTA (E) and THG (F) in paraffin-embedded sections of 5 × 105 NCAM1+ cell grafts. Yellow arrows mark the stained tubule-like structures while orange arrows mark the unstained structures. Images were obtained using a Scion Corporation grayscale digital camera attached to an Olympus BX51.
G,H. Segment-specific IF staining of DBA (G) and LTA (H) in paraffin-embedded sections of 5 × 105 NCAM1+ cell grafts. Hoechst 33342 (blue) was used for nuclear staining. Images were obtained using an Olympus AX70 motorized microscope and spectral unmixing which allows distinction of background from specific staining, using a multispectral imaging system (NuanceFX camera and software).
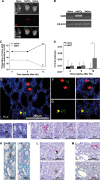
A,B. hNPCs engraft into 5/6 Nx mouse kidneys: (A) hNPCs were labelled with CM-Dil Cell Tracker and injected directly into the renal parenchyma. Mice injected with saline served as controls. Two weeks after cell injection the kidneys were analysed by the maestroCRI in vivo imaging system. Shown are bright field (BF), fluorescence field in red (FF) and merged images of a healthy kidney (naïve, left), remnant kidney treated with hNPCs (middle) or with saline (right). (B) RT-PCR for human-specific β2-microglobulin (hβ2m) demonstrates a representative gel electrophoresis with amplification in the hNPC-injected kidneys, but not in untreated (naïve) or saline-injected kidneys. Mouse β-actin (mβ-actin) served as endogenous control.
C,D. hNPCs halt disease progression and improve CrCl: (C) Injection of hNPCs into 5/6 Nx mice led to a significant decrease in the proportion of mice with >15% of renal function deterioration, compared to saline-treated mice. (D) hNPC-treated mice show a significant positive change in CrCl values at 12 weeks when compared to baseline levels (ΔT CrCl) in contrast to saline-treated mice. *p-Value <0.01.
E–M. hNPCs integrate into existing tubules and regenerate new tubules: Injected remnant kidneys were stained for the human HLA marker (E–G, J, K—upper panel and M) and a human-specific epithelial marker, panCK/MNF116 (H–L). hNPCs integrated into tubules of host mouse (arrows) and regenerated new tubule-like structures (arrowheads), as demonstrated by IF HLA staining (E). Higher magnification of e is shown in (F and G) HLA staining (green, left) merged with DAPI (overlay, right). (H and I) Various modes of engraftment are highlighted: integration within tubules (H; panCK), integration between tubules (I; panCK) and tubular regeneration (J–M representing consecutive sections). (J) Small early tubules stained for HLA and panCK but not for NCAM1. (K) Mature tubules stained for HLA and panCK. (L) A human panCK expressing tubule (upper image) is further delineated to express the human-specific distal marker, EMA (lower image) (M) Staining for HLA (upper image) and cross-reactive proximal marker ENPEP (lower image) shows human origin of a regenerating proximal tubule type.
References
-
- Almeida-Porada G, El Shabrawy D, Porada C, Zanjani ED. Differentiative potential of human metanephric mesenchymal cells. Exp Hematol. 2002;30:1454–1462. - PubMed
-
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol. 2008;313:234–245. - PMC - PubMed
-
- Bruno S, Bussolati B, Grange C, Collino F, di Cantogno LV, Herrera MB, Biancone L, Tetta C, Segoloni G, Camussi G. Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Stem Cells Dev. 2009;18:867–880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

