Regulation of luminal acidification by the V-ATPase
- PMID: 23997191
- PMCID: PMC3768094
- DOI: 10.1152/physiol.00007.2013
Regulation of luminal acidification by the V-ATPase
Abstract
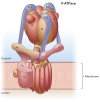
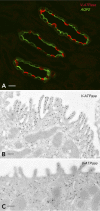
Specialized cells in the body express high levels of V-ATPase in their plasma membrane and respond to hormonal and nonhormonal cues to regulate extracellular acidification. Mutations in or loss of some V-ATPase subunits cause several disorders, including renal distal tubular acidosis and male infertility. This review focuses on the regulation of V-ATPase-dependent luminal acidification in renal intercalated cells and epididymal clear cells, which are key players in these physiological processes.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures


References
-
- Al-Awqati Q. Plasticity in epithelial polarity of renal intercalated cells: targeting of the H+-ATPase and band 3. Am J Physiol Cell Physiol 270: C1571–C1580, 1996 - PubMed
-
- Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 - PMC - PubMed
-
- Banerjee A, Shih T, Alexander EA, Schwartz JH. SNARE proteins regulate H+-ATPase redistribution to the apical membrane in rat renal inner medullary collecting duct cells. J Biol Chem 274: 26518–26522, 1999 - PubMed
-
- Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+ATPase (V-ATPase) recycling. J Biol Chem 280: 8452–8463, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-042956/DK/NIDDK NIH HHS/United States
- DK-097124/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- DK43351/DK/NIDDK NIH HHS/United States
- DK-038452/DK/NIDDK NIH HHS/United States
- R56 DK042956/DK/NIDDK NIH HHS/United States
- R37 DK042956/DK/NIDDK NIH HHS/United States
- R01 DK042956/DK/NIDDK NIH HHS/United States
- R01 DK097124/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- R01 HD040793/HD/NICHD NIH HHS/United States
- HD-040793/HD/NICHD NIH HHS/United States
- P01 DK038452/DK/NIDDK NIH HHS/United States
- DK57521/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

