Designing functional metalloproteins: from structural to catalytic metal sites
- PMID: 23997273
- PMCID: PMC3756834
- DOI: 10.1016/j.ccr.2013.02.007
Designing functional metalloproteins: from structural to catalytic metal sites
Abstract
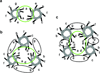
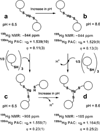
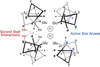
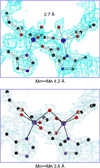
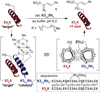
Metalloenzymes efficiently catalyze some of the most important and difficult reactions in nature. For many years, coordination chemists have effectively used small molecule models to understand these systems. More recently, protein design has been shown to be an effective approach for mimicking metal coordination environments. Since the first designed proteins were reported, much success has been seen for incorporating metal sites into proteins and attaining the desired coordination environment but until recently, this has been with a lack of significant catalytic activity. Now there are examples of designed metalloproteins that, although not yet reaching the activity of native enzymes, are considerably closer. In this review, we highlight work leading up to the design of a small metalloprotein containing two metal sites, one for structural stability (HgS3) and the other a separate catalytic zinc site to mimic carbonic anhydrase activity (ZnN3O). The first section will describe previous studies that allowed for a high affinity thiolate site that binds heavy metals in a way that stabilizes three-stranded coiled coils. The second section will examine ways of preparing histidine rich environments that lead to metal based hydrolytic catalysts. We will also discuss other recent examples of the design of structural metal sites and functional metalloenzymes. Our work demonstrates that attaining the proper first coordination geometry of a metal site can lead to a significant fraction of catalytic activity, apparently independent of the type of secondary structure of the surrounding protein environment. We are now in a position to begin to meet the challenge of building a metalloenzyme systematically from the bottom-up by engineering and analyzing interactions directly around the metal site and beyond.
Keywords: Hg thiolates; Zn hydrolase; de novo metalloprotein design.
Figures

















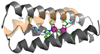

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
