Nonporous Silica Nanoparticles for Nanomedicine Application
- PMID: 23997809
- PMCID: PMC3757135
- DOI: 10.1016/j.nantod.2013.04.007
Nonporous Silica Nanoparticles for Nanomedicine Application
Abstract
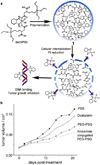
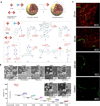
Nanomedicine, the use of nanotechnology for biomedical applications, has potential to change the landscape of the diagnosis and therapy of many diseases. In the past several decades, the advancement in nanotechnology and material science has resulted in a large number of organic and inorganic nanomedicine platforms. Silica nanoparticles (NPs), which exhibit many unique properties, offer a promising drug delivery platform to realize the potential of nanomedicine. Mesoporous silica NPs have been extensively reviewed previously. Here we review the current state of the development and application of nonporous silica NPs for drug delivery and molecular imaging.
Keywords: Nanomedicine; biomaterials; drug delivery; gene delivery; molecular imaging; nanotoxicity; nonporous silica nanoparticles.
Figures




References
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat. Nanotechnol. 2007;2:751–760. - PubMed
-
- Petros RA, DeSimone JM. Nat. Rev. Drug Discov. 2010;9:615–627. - PubMed
-
- Duncan R. Nat. Rev. Cancer. 2006;6:688–701. - PubMed
-
- Davis ME, Chen Z, Shin DM. Nat. Rev. Drug Discov. 2008;7:771–782. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
