Magnetic resonance monitoring of lesion evolution in multiple sclerosis
- PMID: 23997815
- PMCID: PMC3755529
- DOI: 10.1177/1756285613484079
Magnetic resonance monitoring of lesion evolution in multiple sclerosis
Abstract
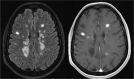
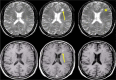
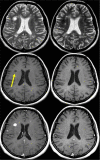
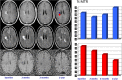
Disease activity in multiple sclerosis (MS) is strongly linked to the formation of new lesions, which involves a complex sequence of inflammatory, degenerative, and reparative processes. Conventional magnetic resonance imaging (MRI) techniques, such as T2-weighted and gadolinium-enhanced T1-weighted sequences, are highly sensitive in demonstrating the spatial and temporal dissemination of demyelinating plaques in the brain and spinal cord. Hence, these techniques can provide quantitative assessment of disease activity in patients with MS, and they are commonly used in monitoring treatment efficacy in clinical trials and in individual cases. However, the correlation between conventional MRI measures of disease activity and the clinical manifestations of the disease, particularly irreversible disability, is weak. This has been explained by a process of exhaustion of both structural and functional redundancies that increasingly prevents repair and recovery, and by the fact that these imaging techniques do not suffice to explain the entire spectrum of the disease process and lesion development. Nonconventional MRI techniques, such as magnetization transfer imaging, diffusion-weighted imaging, and proton magnetic resonance spectroscopy, which can selectively measure the more destructive aspects of MS pathology and monitor the reparative mechanisms of this disease, are increasingly being used for serial analysis of new lesion formation and provide a better approximation of the pathological substrate of MS plaques. These nonconventional MRI-based measures better assess the serial changes in newly forming lesions and improve our understanding of the relationship between the damaging and reparative mechanisms that occur in MS.
Keywords: diffusion-weighted imaging; lesion development; magnetic resonance imaging; magnetization transfer imaging; multiple sclerosis; proton magnetic resonance spectroscopy.
Conflict of interest statement
Figures


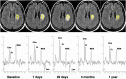
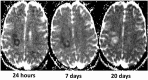
References
-
- Arnold D., De Stefano N., Narayanan S., Matthews P. (2000) Proton MR spectroscopy in multiple sclerosis. Neuroimaging Clin N Am 10: 789–798 - PubMed
-
- Arnold D., Matthews P., Francis G., O’Connor J., Antel J. (1992) Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol 31: 235–241 - PubMed
-
- Barkhof F. (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15: 239–245 - PubMed
-
- Barkhof F., Calabresi P., Miller D., Reingold S.(2009) Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 5: 256–266 - PubMed
-
- Budde M., Kim J., Liang H., Schmidt R., Russell J., Cross A., et al. (2007) Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 57: 688–695 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

