Therapeutic advances in the management of Pompe disease and other metabolic myopathies
- PMID: 23997816
- PMCID: PMC3755530
- DOI: 10.1177/1756285613487570
Therapeutic advances in the management of Pompe disease and other metabolic myopathies
Abstract
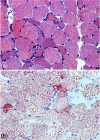
The world of metabolic myopathies has been dramatically modified by the advent of enzyme replacement therapy (ERT), the first causative treatment for glycogenosis type II (GSDII) or Pompe disease, which has given new impetus to research into that disease and also other pathologies. This article reviews new advances in the treatment of GSDII, the consensus about ERT, and its limitations. In addition, the most recent knowledge regarding the pathophysiology, phenotype, and genotype of the disease is discussed. Pharmacological, immunotherapy, nutritional, and physical/rehabilitative treatments for late-onset Pompe disease and other metabolic myopathies are covered, including treatments for defects in glycogen metabolism, such as glycogenosis type V (McArdle disease), and glycogenosis type III (debrancher enzyme deficiency), and defects in lipid metabolism, such as carnitine palmitoyltransferase II deficiency and electron transferring flavoprotein dehydrogenase deficiency, or riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency.
Keywords: CPT2 deficiency; Glycogenosis type II; McArdle disease; RR-MADD; glycogenosis type III.
Conflict of interest statement
Figures

References
-
- Andersen S., Haller R., Vissing J. (2008) Effect of oral sucrose shortly before exercise on work capacity in McArdle disease. Arch Neurol 65: 786–789 - PubMed
-
- Andersen S., Vissing J. (2008) Carbohydrate- and protein-rich diets in McArdle disease: effects on exercise capacity. J Neurol Neurosurg Psychiatry 79: 1359–1363 [Erratum (2010): 81: 1414] - PubMed
-
- Angelini C., Engel A. (1972) Comparative study of acid maltase deficiency. Biochemical differences between infantile, childhood, and adult types. Arch Neurol 26: 344–349 - PubMed
-
- Angelini C., Semplicini C., Ravaglia S., Bembi B., Servidei S., Pegoraro E., et al. (2012a) Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J Neurol 259: 952–958 - PubMed
-
- Angelini C., Semplicini C., Ravaglia S., Moggio M., Comi G., Musumeci O., et al. (2012b) New motor outcome function measures in evaluation of late-onset Pompe disease before and after enzyme replacement therapy. Muscle Nerve 45: 831–834 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

