Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC)
- PMID: 23997985
- PMCID: PMC3755522
Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC)
Abstract
Background: Several studies revealed that MSC from human bone marrow can downregulate graft-versus-host disease (GVHD) after allogeneic HSCT.
Methods: Herein we present 50 patients with acute GVHD who got 74 (1-4) MSC infusions for 54 separate episodes of aGVHD.
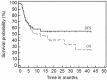
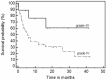
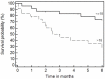
Results: aGVHD was defined as steroid resistant grade IV aGVHD in 42 cases. The major presentation was gastrointestinal GVHD; two (n=18) or more (n=21) systems were involved in the majority of cases. The 1(st) infusion with MSC was given on day +27 (range, 1 to 136); d+45 (range, +11 to +150) post diagnosis of aGVHD and HSCT, respectively. In 2/3 of the cases treatment was performed with frozen stocked MSCs; in 62 cases early passages (1-3) were used. The median number of infused cells was 1.14±0.47 million per kg in the first injection and up to 4.27 (1.70±1.10) millions in total. The two patients with aggressive liver GVHD received MSCs injections intra hepatic arteries without changes of blood flow or evidence cytolysis, but also without a visible effect. Disease free survival at 3.6 years was 56%. We observed better overall survival in patients with GVHD grade < 4, in responders to the 1(st) treatment with MSC, and in pediatric group. The multivariate analysis demonstrated independent influence on survival of initial response and younger age. There were no immediate or late toxicity or side effects.
Conclusion: Injection of MSCs seems to be a promising and safe treatment of GVHD. The encouraging results obviously should be confirmed in a randomized prospective study.
Keywords: Mesenchymal stromal cells (MSC); graft versus host disease; hematopoietic stem cell transplantation; mesenchymal stem cells; steroid resistance.
Figures


References
-
- Prockop DJ. Marrow stomal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Caplan Al. The mesengenic process. Clin Plast Surg. 1994;21:429–435. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
