Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice
- PMID: 23999092
- PMCID: PMC3814374
- DOI: 10.1093/nar/gkt780
Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice
Abstract
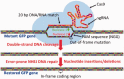
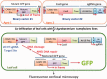
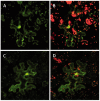
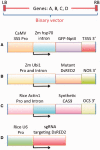
The type II CRISPR/Cas system from Streptococcus pyogenes and its simplified derivative, the Cas9/single guide RNA (sgRNA) system, have emerged as potent new tools for targeted gene knockout in bacteria, yeast, fruit fly, zebrafish and human cells. Here, we describe adaptations of these systems leading to successful expression of the Cas9/sgRNA system in two dicot plant species, Arabidopsis and tobacco, and two monocot crop species, rice and sorghum. Agrobacterium tumefaciens was used for delivery of genes encoding Cas9, sgRNA and a non-fuctional, mutant green fluorescence protein (GFP) to Arabidopsis and tobacco. The mutant GFP gene contained target sites in its 5' coding regions that were successfully cleaved by a CAS9/sgRNA complex that, along with error-prone DNA repair, resulted in creation of functional GFP genes. DNA sequencing confirmed Cas9/sgRNA-mediated mutagenesis at the target site. Rice protoplast cells transformed with Cas9/sgRNA constructs targeting the promoter region of the bacterial blight susceptibility genes, OsSWEET14 and OsSWEET11, were confirmed by DNA sequencing to contain mutated DNA sequences at the target sites. Successful demonstration of the Cas9/sgRNA system in model plant and crop species bodes well for its near-term use as a facile and powerful means of plant genetic engineering for scientific and agricultural applications.
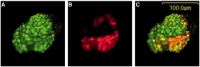
Figures



References
-
- Smithies O, Gregg RG, Boggs SS, Doralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985;317:230–234. - PubMed
-
- Capecchi MR, Thomas KR, Folger KR. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986;44:419–428. - PubMed
-
- Capecchi MR. Generating mice with targeted mutations. Nat. Med. 2001;7:1086–1090. - PubMed
-
- Domínguez-López S, Howell R, Gobbi G. Characterization of serotonin neurotransmission in knockout mice: implications for major depression. Rev. Neurosci. 2012;23:429–443. - PubMed
-
- van der Weyden L, Adams DJ. Cancer of mice and men: old twists and new tails. J. Pathol. 2013;230:4–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

