HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations
- PMID: 23999440
- PMCID: PMC3754249
- DOI: 10.1172/JCI67230
HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations
Abstract
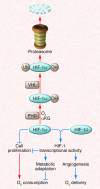
Hypoxia occurs frequently in human cancers and induces adaptive changes in cell metabolism that include a switch from oxidative phosphorylation to glycolysis, increased glycogen synthesis, and a switch from glucose to glutamine as the major substrate for fatty acid synthesis. This broad metabolic reprogramming is coordinated at the transcriptional level by HIF-1, which functions as a master regulator to balance oxygen supply and demand. HIF-1 is also activated in cancer cells by tumor suppressor (e.g., VHL) loss of function and oncogene gain of function (leading to PI3K/AKT/mTOR activity) and mediates metabolic alterations that drive cancer progression and resistance to therapy. Inhibitors of HIF-1 or metabolic enzymes may impair the metabolic flexibility of cancer cells and make them more sensitive to anticancer drugs.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

