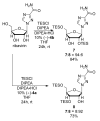
Practical silyl protection of ribonucleosides
- PMID: 24000770
- PMCID: PMC3857215
- DOI: 10.1021/ol402023c
Practical silyl protection of ribonucleosides
Abstract
Herein we report the site-selective silylation of the ribonucelosides. The method enables a simple and efficient procedure for accessing suitably protected monomers for automated RNA synthesis. Switching to the opposite enantiomer of the catalyst allows for the selective silylation of the 3'-hydroxyl, which could be used in the synthesis of unnatural RNA or for the analoging of ribonucelosides. Lastly, the procedure was extended to ribavirin a potent antiviral therapeutic.
Figures
Similar articles
-
Synthesis of 5'-O-DMT-2'-O-TBS Mononucleosides Using an Organic Catalyst.Curr Protoc Nucleic Acid Chem. 2014 Jun 24;57:2.17.1-11. doi: 10.1002/0471142700.nc0217s57. Curr Protoc Nucleic Acid Chem. 2014. PMID: 24961720 Free PMC article. Review.
-
[Acyclic analogs of ribavirin. Synthesis and antiviral activity].Bioorg Khim. 1988 May;14(5):689-93. Bioorg Khim. 1988. PMID: 3422011 Russian.
-
Synthesis and antiviral evaluation of a mutagenic and non-hydrogen bonding ribonucleoside analogue: 1-beta-D-Ribofuranosyl-3-nitropyrrole.Biochemistry. 2002 Jul 23;41(29):9026-33. doi: 10.1021/bi026120w. Biochemistry. 2002. PMID: 12119016
-
Ribonucleosides bearing Michael acceptors: synthesis, molecular docking, enzyme inhibition data and antiviral activity.Nucleic Acids Symp Ser (Oxf). 2008;(52):625-6. doi: 10.1093/nass/nrn316. Nucleic Acids Symp Ser (Oxf). 2008. PMID: 18776535 No abstract available.
-
[Ribavirin as an antiviral agent: chemistry, molecular mechanism of action and possibilities of use (review)].Vopr Med Khim. 1986 Jan-Feb;32(1):10-9. Vopr Med Khim. 1986. PMID: 3513441 Review. Russian. No abstract available.
Cited by
-
Site-selective redox isomerizations of furanosides using a combined arylboronic acid/photoredox catalyst system.Chem Sci. 2020 Jan 3;11(6):1531-1537. doi: 10.1039/c9sc05173b. Chem Sci. 2020. PMID: 34084383 Free PMC article.
-
A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol.Nat Chem. 2023 Mar;15(3):424-435. doi: 10.1038/s41557-022-01110-z. Epub 2022 Dec 30. Nat Chem. 2023. PMID: 36585443 Free PMC article.
-
Exploiting non-covalent interactions in selective carbohydrate synthesis.Nat Rev Chem. 2021 Nov;5(11):792-815. doi: 10.1038/s41570-021-00324-y. Epub 2021 Oct 6. Nat Rev Chem. 2021. PMID: 37117666 Review.
-
10th anniversary of discovering cGAMP: synthesis and beyond.Org Chem Front. 2023 Feb 21;10(4):1086-1098. doi: 10.1039/d2qo02033e. Epub 2023 Jan 25. Org Chem Front. 2023. PMID: 37144138 Free PMC article.
-
Synthesis of 5'-O-DMT-2'-O-TBS Mononucleosides Using an Organic Catalyst.Curr Protoc Nucleic Acid Chem. 2014 Jun 24;57:2.17.1-11. doi: 10.1002/0471142700.nc0217s57. Curr Protoc Nucleic Acid Chem. 2014. PMID: 24961720 Free PMC article. Review.
References
-
- Mahatthananchai J, Dumas AM, Bode JW. Angew. Chem., Int. Ed. 2012;51:10954–10990. - PubMed
-
- Lewis CA, Miller SJ. Angew. Chem., Int. Ed. 2006;45:5616–5619. - PubMed
- Chen MS, White MC. Science. 2007;318:783–787. - PubMed
- Lewis CA, Longcore KE, Miller SJ, Wender PA. J. Nat. Prod. 2009;72:1864–1869. - PMC - PubMed
- Yoshida K, Furuta T, Kawabata T. Tetrahedron Lett. 2010;51:4830–4832.
- Fowler BS, Laemmerhold KM, Miller SJ. J. Am. Chem. Soc. 2012;134:9755–9761. - PMC - PubMed
- Pathak TP, Miller SJ. J. Am. Chem. Soc. 2012;134:6120–6123. - PMC - PubMed
- Bruckl T, Baxter RD, Ishihara Y, Baran PS. Acc. Chem. Res. 2012;45:826–839. - PMC - PubMed
- Beale TM, Taylor MS. Org. Lett. 2013;15:1358–1361. - PubMed
- Pathak TP, Miller SJ. J. Am. Chem. Soc. 2013;135:8415–8422. - PMC - PubMed
-
-
For examples of directed and reagent controled functionalization of natural products see: Breslow R, Baldwin S, Flechtne T, Kalicky P, Liu S, Washburn W. J. Am. Chem. Soc. 1973;95:3251–3262. Breslow R, Corcoran RJ, Snider BB, Doll RJ, Khanna PL, Kaleya R. J. Am. Chem. Soc. 1977;99:905–915. Breslow R, Heyer D. J. Am. Chem. Soc. 1982;104:2045–2046. Wilcock BC, Uno BE, Bromann GL, Clark MJ, Anderson TM, Burke MD. Nat. Chem. 2012;4:996–1003.
-
-
-
For a review on sugar functionalization see: Lee D, Taylor MS. Synthesis. 2012;44:3421–3431.
-
-
- Griswold KS, Miller SJ. Tetrahedron. 2003;59:8869–8875.
- Kawabata T, Muramatsu W, Nishio T, Shibata T, Schedel H. J. Am. Chem. Soc. 2007;129:12890–12895. - PubMed
- Kawabata T, Furuta T. Chem. Lett. 2009;38:640–647.
- Chan L, Taylor MS. Org. Lett. 2011;13:3090–3093. - PubMed
- Gouliaras C, Lee D, Chan L, Taylor MS. J. Am. Chem. Soc. 2011;133:13926–13929. - PubMed
- Lee D, Taylor MS. J. Am. Chem. Soc. 2011;133:3724–3727. - PubMed
- Lee D, Williamson CL, Chan L, Taylor MS. J. Am. Chem. Soc. 2012;134:8260–8267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources