A highly stereoselective Diels-Alder cycloaddition of enones with chiral cyclic 2-amidodienes derived from allenamides
- PMID: 24001055
- PMCID: PMC3823681
- DOI: 10.1021/ol402254p
A highly stereoselective Diels-Alder cycloaddition of enones with chiral cyclic 2-amidodienes derived from allenamides
Abstract
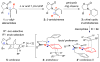
Lewis acid promoted Diels-Alder cycloadditions of a series of de novo chiral cyclic 2-amidodienes are described. These cyclic 2-amidodienes are derived from chiral α-allyl allenamides via a sequence of E-selective 1,3-H shift and 6π-electron pericyclic ring closure. With enones serving as effective dienophiles, these cycloadditions can be highly diastereoselective depending upon the chiral amide substituent, thereby representing a facile entry to optically enriched [2.2.2]bicyclic manifolds.
Figures
References
-
- Hayashi R, Hsung RP, Feltenberger JB, Lohse AG. Org Lett. 2009;11:2125. - PMC - PubMed
- Hayashi R, Feltenberger JB, Hsung RP. Org Lett. 2010;12:1152. - PMC - PubMed
- Hayashi R, Walton MC, Hsung RP, Schwab J, Yu X. Org Lett. 2010;12:5768. - PMC - PubMed
- Hayashi R, Feltenberger JB, Lohse AG, Walton MC, Hsung RP. Beil J Org Chem. 2011;7:410. - PMC - PubMed
-
-
For leading reviews on allenamide chemistry, see: Lu T, Lu Z, Ma ZX, Zhang Y, Hsung RP. Chem Rev. 2013;130:4862.Hsung RP, Wei LL, Xiong H. Acc Chem Res. 2003;36:773.
-
-
-
Also see: Standen PE, Kimber MC. Curr Opin Drug Discov & Devel. 2010;13:645.Deagostino A, Prandi C, Tabasso S, Venturello P. Molecules. 2010;15:2667.
-
-
-
For general reviews on allenes, see: Krause N, Hashmi ASK. Modern Allene Chemistry. 1 and 2 Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004.
-
-
-
For reviews on chemistry of dienamides, see: Overman LE. Acc Chem Res. 1980;13:218.Petrzilka M. Synthesis. 1981:753.Campbell AL, Lenz GR. Synthesis. 1987:421.Krohn K. Angew Chem Int Ed Engl. 1993;32:1582.Enders D, Meyer O. Liebigs Ann. 1996:1023.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources