Temporal and spatial variation in Anaplasma phagocytophilum infection in Swedish moose (Alces alces)
- PMID: 24001524
- PMCID: PMC4045167
- DOI: 10.1017/S0950268813002094
Temporal and spatial variation in Anaplasma phagocytophilum infection in Swedish moose (Alces alces)
Abstract
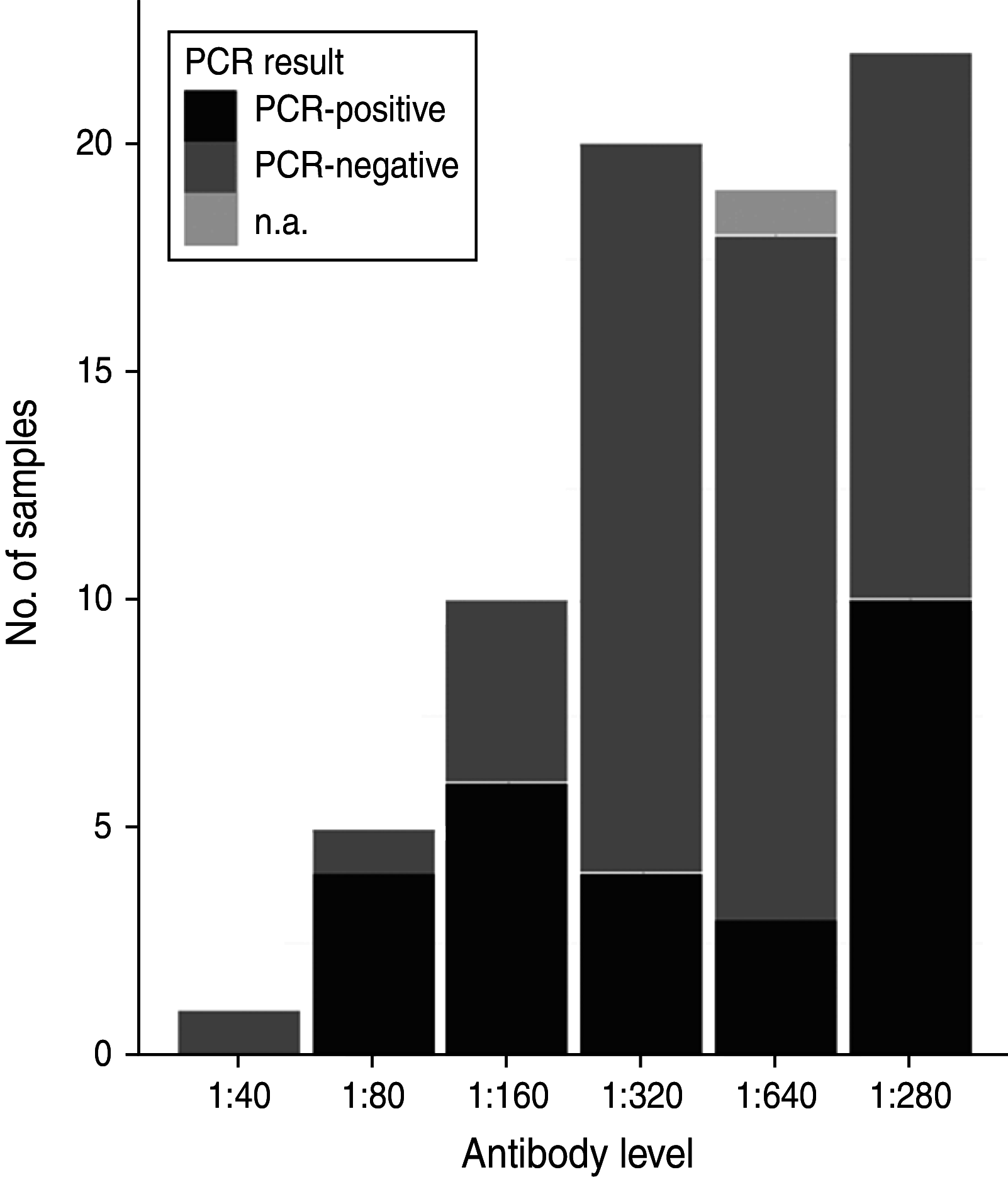
The occurrence of Anaplasma phagocytophilum was investigated in spleen and serum samples from Swedish moose (Alces alces) in southern Sweden (island and mainland). Samples were analysed for presence of A. phagocytophilum DNA by real-time PCR (n = 263), and for Anaplasma antibodies with ELISA serology (n = 234). All serum samples had antibodies against A. phagocytophilum. The mean DNA-based prevalence was 26·3%, and significant (P < 0·01) temporal, and spatial variation was found. Island moose had significantly (P < 0·001) higher prevalence of A. phagocytophilum DNA than moose from the mainland areas. Two samples were sequenced to determine genetic variation in the 16S rRNA and groESL genes. Genetic sequence similarity with the human granulocytic anaplasmosis agent, equine granulocytic ehrlichiosis agent, and different wildlife-associated A. phagocytophilum variants were observed in the 16S rRNA and groESL genes. Our study shows that moose are exposed to A. phagocytophilum in Sweden, and represent a potential wildlife reservoir of the pathogen.
Figures
Similar articles
-
Anaplasma phagocytophilum infection in moose (Alces alces) in Norway.Microbes Infect. 2015 Nov-Dec;17(11-12):823-8. doi: 10.1016/j.micinf.2015.09.013. Epub 2015 Sep 30. Microbes Infect. 2015. PMID: 26428857
-
Impact of tick-borne Anaplasma phagocytophilum infections in calves of moose (Alces alces) in southern Norway.Folia Parasitol (Praha). 2021 Oct 13;68:2021.023. doi: 10.14411/fp.2021.023. Folia Parasitol (Praha). 2021. PMID: 34782490
-
The infection of reintroduced ruminants - Bison bonasus and Alces alces - with Anaplasma phagocytophilum in northern Poland.Acta Parasitol. 2015 Dec;60(4):645-8. doi: 10.1515/ap-2015-0091. Acta Parasitol. 2015. PMID: 26408585
-
[Study of the heterogeneity of 16s rRNA gene and groESL operone in the dna samples of Anaplasma phagocytophilum, Ehrlichia muris, and "Candidatus Neoehrlichia mikurensis" determined in the Ixodes persulcatus ticks in the area of Urals, Siberia, and far east of Russia].Mol Gen Mikrobiol Virusol. 2011;(2):17-23. Mol Gen Mikrobiol Virusol. 2011. PMID: 21786632 Russian.
-
Epidemiology, genetic variants and clinical course of natural infections with Anaplasma phagocytophilum in a dairy cattle herd.Parasit Vectors. 2018 Jan 8;11(1):20. doi: 10.1186/s13071-017-2570-1. Parasit Vectors. 2018. PMID: 29310697 Free PMC article.
Cited by
-
Prevalence and Genotyping of Anaplasma phagocytophilum Strains from Wild Animals, European Bison (Bison bonasus) and Eurasian Moose (Alces alces) in Poland.Animals (Basel). 2022 May 9;12(9):1222. doi: 10.3390/ani12091222. Animals (Basel). 2022. PMID: 35565648 Free PMC article.
-
Survey of selected tick-borne diseases in dogs in Finland.Parasit Vectors. 2014 Jun 23;7:285. doi: 10.1186/1756-3305-7-285. Parasit Vectors. 2014. PMID: 24957468 Free PMC article.
-
Common and atypical presentations of Anaplasma phagocytophilum infection in equids with emphasis on neurologic and muscle disease.J Vet Intern Med. 2024 Jan-Feb;38(1):440-448. doi: 10.1111/jvim.16964. Epub 2023 Dec 1. J Vet Intern Med. 2024. PMID: 38038253 Free PMC article.
-
A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe.Parasit Vectors. 2019 Dec 21;12(1):599. doi: 10.1186/s13071-019-3852-6. Parasit Vectors. 2019. PMID: 31864403 Free PMC article. Review.
-
Vertical transmission of Bartonella schoenbuchensis in Lipoptena cervi.Parasit Vectors. 2015 Mar 21;8:176. doi: 10.1186/s13071-015-0764-y. Parasit Vectors. 2015. PMID: 25889985 Free PMC article.
References
-
- Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Veterinary Parasitology 2010; 167: 108–122. - PubMed
-
- Teglas MB, Foley J. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American tick vector species, Ixodes pacificus and Ixodes scapularis (Acari: Ixodidae). Experimental & Applied Accarology 2006; 38: 47–58. - PubMed
-
- Gribble DH. Equine ehrlichiosis. Journal of the American Veterinary Medical Association 1969; 155: 462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

