Pharmacokinetic and pharmacodynamic considerations in antimalarial dose optimization
- PMID: 24002099
- PMCID: PMC3837842
- DOI: 10.1128/AAC.00287-13
Pharmacokinetic and pharmacodynamic considerations in antimalarial dose optimization
Abstract
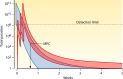
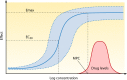
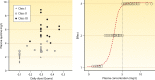
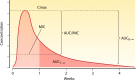
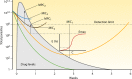
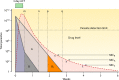
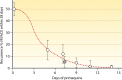
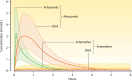
Antimalarial drugs have usually been first deployed in areas of malaria endemicity at doses which were too low, particularly for high-risk groups such as young children and pregnant women. This may accelerate the emergence and spread of resistance, thereby shortening the useful life of the drug, but it is an inevitable consequence of the current imprecise method of dose finding. An alternative approach to dose finding is suggested in which phase 2 studies concentrate initially on pharmacokinetic-pharmacodynamic (PK-PD) characterization and in vivo calibration of in vitro susceptibility information. PD assessment is facilitated in malaria because serial parasite densities are readily assessed by microscopy, and at low densities by quantitative PCR, so that initial therapeutic responses can be quantitated accurately. If the in vivo MIC could be characterized early in phase 2 studies, it would provide a sound basis for the choice of dose in all target populations in subsequent combination treatments. Population PK assessments in phase 2b and phase 3 studies which characterize PK differences between different age groups, clinical disease states, and human populations can then be combined with the PK-PD observations to provide a sound evidence base for dose recommendations in different target groups.
Figures


References
-
- World Health Organization 2010. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland
-
- Ashley EA, Pinoges L, Turyakira E, Dorsey G, Checchi F, Bukirwa H, van den Broek I, Zongo I, Urruta PP, van Herp M, Balkan S, Taylor WR, Olliaro P, Guthmann JP. 2008. Different methodological approaches to the assessment of in vivo efficacy of three artemisinin-based combination antimalarial treatments for the treatment of uncomplicated falciparum malaria in African children. Malar. J. 7:154.10.1186/1475-2875-7-154 - DOI - PMC - PubMed
-
- World Health Organization 2003. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. World Health Organization, Geneva, Switzerland
-
- Barnes KI, Little F, Smith PJ, Evans A, Watkins WM, White NJ. 2006. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin. Pharmacol. Ther. 80:582–596 - PubMed
-
- Baird JK, Hoffman SL. 2004. Primaquine therapy for malaria. Clin. Infect. Dis. 39:1336–1345 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

