Heparin blocks transfer of extracellular vesicles between donor and recipient cells
- PMID: 24002181
- PMCID: PMC3856724
- DOI: 10.1007/s11060-013-1235-y
Heparin blocks transfer of extracellular vesicles between donor and recipient cells
Abstract
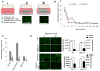
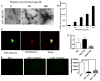
Extracellular vesicles (EVs) have been implicated in tumorigenesis. Biomolecules which can block EV binding and uptake into recipient cells may be of therapeutic value as well as enhance understanding of EV biology. Here, we show that heparin interacts with uptake of tumor-derived as well as non-tumor-derived EVs into recipient cells. Incubation of glioma cell-derived EVs with heparin resulted in micron-sized structures observed by transmission electron microscopy, with EVs clearly visible within these structures. Inclusion of heparin greatly diminished transfer of labeled EVs from donor to recipient tumor cells. We also show a direct interaction between heparin and EVs using confocal microscopy. We found that the block in EV uptake was at the level of cell binding and not internalization. Finally, incubation of glioma-derived EVs containing EGFRvIII mRNA with heparin reduced transfer of this message to recipient cells. The effect of heparin on EVs uptake may provide a unique tool to study EV function. It may also foster research of heparin or its derivatives as a therapeutic for disease in which EVs play a role.
Figures

References
-
- Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–2018. - PubMed
-
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. - PubMed
-
- Chaput N, Taieb J, Andre F, Zitvogel L. The potential of exosomes in immunotherapy. Expert Opin Biol Ther. 2005;5:737–747. - PubMed
-
- Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36(1–3):247–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

