Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study
- PMID: 24002500
- PMCID: PMC4879693
- DOI: 10.1200/JCO.2013.49.2835
Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study
Abstract
Purpose: Although dose-intensive strategies or high-dose therapy induction followed by autologous stem-cell transplantation have improved the outcome for patients with mantle-cell lymphoma (MCL), most eventually relapse and subsequently respond poorly to additional therapy. Bortezomib (in the United States) and temsirolimus (in Europe) are currently the only two treatments approved for relapsed disease. Lenalidomide is an immunomodulatory agent with proven tumoricidal and antiproliferative activity in MCL. The MCL-001 (EMERGE) trial is a global, multicenter phase II study examining the safety and efficacy of lenalidomide in patients who had relapsed or were refractory to bortezomib.
Patients and methods: Lenalidomide 25 mg orally was administered on days 1 through 21 every 28 days until disease progression or intolerance. Primary end points were overall response rate (ORR) and duration of response (DOR); secondary end points included complete response (CR) rate, progression-free survival (PFS), overall survival (OS), and safety.
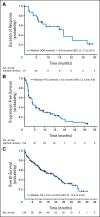
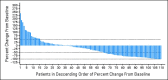
Results: In all, 134 patients were enrolled with a median age of 67 years and a median of four prior therapies (range, two to 10 prior therapies). The ORR was 28% (7.5% CR/CR unconfirmed) with rapid time to response (median, 2.2 months) and a median DOR of 16.6 months (95% CI, 7.7 to 26.7 months). Median PFS was 4.0 months (95% CI, 3.6 to 5.6 months), and median OS was 19.0 months (95% CI, 12.5 to 23.9 months). The most common grade 3 to 4 adverse events were neutropenia (43%), thrombocytopenia (28%), anemia (11%), pneumonia (8%), and fatigue (7%).
Conclusion: The MCL-001 study demonstrated durable efficacy of lenalidomide with a predictable safety profile in heavily pretreated patients with MCL who had all relapsed or progressed after or were refractory to bortezomib.
Trial registration: ClinicalTrials.gov NCT00737529.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Armitage JO. Treatment of non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1023–1030. - PubMed
-
- Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113:791–798. - PubMed
-
- Turner JJ, Hughes AM, Kricker A, et al. WHO non-Hodgkin's lymphoma classification by criterion-based report review followed by targeted pathology review: An effective strategy for epidemiology studies. Cancer Epidemiol Biomarkers Prev. 2005;14:2213–2219. - PubMed
-
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma: The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [No authors listed] - PubMed
-
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes—Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical