Contraindicated use of bevacizumab and toxicity in elderly patients with cancer
- PMID: 24002522
- PMCID: PMC3782151
- DOI: 10.1200/JCO.2012.48.4857
Contraindicated use of bevacizumab and toxicity in elderly patients with cancer
Abstract
Purpose: Drugs are approved on the basis of randomized trials conducted in selected populations. However, once approved, these treatments are usually expanded to patients ineligible for the trial.
Patients and methods: We used the SEER-Medicare database to identify subjects older than 65 years with metastatic breast, lung, and colon cancer, diagnosed between 2004 and 2007 and undergoing follow-up to 2009, who received bevacizumab. We defined a contraindication as having at least two billing claims before bevacizumab for thrombosis, cardiac disease, stroke, hemorrhage, hemoptysis, or GI perforation. We defined toxicity as first development of one of these conditions after therapy.
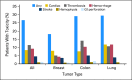
Results: Among 16,085 metastatic patients identified, 3,039 (18.9%) received bevacizumab. Receipt of bevacizumab was associated with white race, later year of diagnosis, tumor type, and decreased comorbid conditions. Of patients who received bevacizumab, 1,082 (35.5%) had a contraindication. In multivariate analysis, receipt of bevacizumab with a contraindication was associated with black race (odds ratio [OR] = 2.6; 95% CI, 1.4 to 4.9), increased age, comorbidity, later year of diagnosis, and lower socioeconomic status. Patients with lung (OR = 1.7; 95% CI, 1.1 to 2.4) and colon cancer (OR = 1.4; 95% CI, 1.1 to 1.9) were more likely to have a contraindication. In the group with no contraindication, 30% had a complication after bevacizumab; black patients were more likely to have a complication than were white patients (OR = 1.9; 95% CI, 1.21 to 2.93).
Conclusion: Our study demonstrates widespread use of bevacizumab among patients who had contraindications. Black patients were less likely to receive the drug, but those who did were more likely to have a contraindication. Efforts to understand toxicity and efficacy in populations excluded from clinical trials are needed.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
-
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources