In vitro hematological and in vivo vasoactivity assessment of dextran functionalized graphene
- PMID: 24002570
- PMCID: PMC3761081
- DOI: 10.1038/srep02584
In vitro hematological and in vivo vasoactivity assessment of dextran functionalized graphene
Abstract
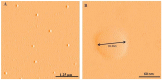
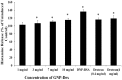
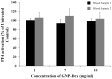
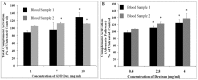
The intravenous, intramuscular or intraperitoneal administration of water solubilized graphene nanoparticles for biomedical applications will result in their interaction with the hematological components and vasculature. Herein, we have investigated the effects of dextran functionalized graphene nanoplatelets (GNP-Dex) on histamine release, platelet activation, immune activation, blood cell hemolysis in vitro, and vasoactivity in vivo. The results indicate that GNP-Dex formulations prevented histamine release from activated RBL-2H3 rat mast cells, and at concentrations ≥ 7 mg/ml, showed a 12-20% increase in levels of complement proteins. Cytokine (TNF-Alpha and IL-10) levels remained within normal range. GNP-Dex formulations did not cause platelet activation or blood cell hemolysis. Using the hamster cheek pouch in vivo model, the initial vasoactivity of GNP-Dex at concentrations (1-50 mg/ml) equivalent to the first pass of a bolus injection was a brief concentration-dependent dilation in arcade and terminal arterioles. However, they did not induce a pro-inflammatory endothelial dysfunction effect.
Figures


Similar articles
-
Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations.Biomaterials. 2014 Aug;35(25):7022-31. doi: 10.1016/j.biomaterials.2014.04.066. Epub 2014 May 20. Biomaterials. 2014. PMID: 24854092 Free PMC article.
-
Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent.Int J Nanomedicine. 2013;8:2821-33. doi: 10.2147/IJN.S47062. Epub 2013 Aug 5. Int J Nanomedicine. 2013. PMID: 23946653 Free PMC article.
-
In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles.Colloids Surf B Biointerfaces. 2015 Oct 1;134:122-30. doi: 10.1016/j.colsurfb.2015.06.046. Epub 2015 Jun 29. Colloids Surf B Biointerfaces. 2015. PMID: 26183082
-
Vasoactive effects of stable aqueous suspensions of single walled carbon nanotubes in hamsters and mice.Nanotoxicology. 2014 Dec;8(8):867-75. doi: 10.3109/17435390.2013.837209. Epub 2013 Sep 30. Nanotoxicology. 2014. PMID: 23992463 Free PMC article.
-
Acetalated dextran based nano- and microparticles: synthesis, fabrication, and therapeutic applications.Chem Commun (Camb). 2021 Apr 29;57(35):4212-4229. doi: 10.1039/d1cc00811k. Chem Commun (Camb). 2021. PMID: 33913978 Review.
Cited by
-
Metabolomic insights of macrophage responses to graphene nanoplatelets: Role of scavenger receptor CD36.PLoS One. 2018 Nov 7;13(11):e0207042. doi: 10.1371/journal.pone.0207042. eCollection 2018. PLoS One. 2018. PMID: 30403754 Free PMC article.
-
Graphene Family Nanomaterials in Ocular Applications: Physicochemical Properties and Toxicity.Chem Res Toxicol. 2021 Jun 21;34(6):1386-1402. doi: 10.1021/acs.chemrestox.0c00340. Epub 2021 May 27. Chem Res Toxicol. 2021. PMID: 34041903 Free PMC article. Review.
-
Long Term Influence of Carbon Nanoparticles on Health and Liver Status in Rats.PLoS One. 2015 Dec 14;10(12):e0144821. doi: 10.1371/journal.pone.0144821. eCollection 2015. PLoS One. 2015. PMID: 26657282 Free PMC article.
-
Safety and Efficacy of A High Performance Graphene-Based Magnetic Resonance Imaging Contrast Agent for Renal Abnormalities.Graphene Technol. 2016 Dec;1(1):17-28. doi: 10.1007/s41127-016-0001-2. Epub 2016 Aug 3. Graphene Technol. 2016. PMID: 28261636 Free PMC article.
-
Efficient skin interactions of graphene derivatives: challenge, opportunity or both?Nanoscale Adv. 2023 Oct 11;5(21):5923-5931. doi: 10.1039/d3na00574g. eCollection 2023 Oct 24. Nanoscale Adv. 2023. PMID: 37881716 Free PMC article.
References
-
- Shao Y. et al. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis. 22, 1027–36 (2010).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

