Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase
- PMID: 24002601
- PMCID: PMC3776971
- DOI: 10.1038/bjc.2013.371
Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase
Abstract
Background: Rituximab and trastuzumab were the first therapeutic monoclonal antibodies (mAbs) approved in oncology. Both antibodies are delivered by the intravenous (IV) route, but recently subcutaneous (SC) formulations have been developed. Subcutaneous administration of mAbs can offer substantial patient and resource benefits compared with IV, but SC administration of some mAbs can be limited by drug volume. Recombinant human hyaluronidase (rHuPH20) temporarily degrades hyaluronan, allowing SC delivery of drug volumes that might not otherwise be feasible.
Methods: Clinical trials assessing coformulation of rituximab or trastuzumab with rHuPH20 for SC administration were reviewed.
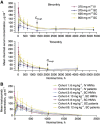
Results: Phase I trials of rituximab SC maintenance therapy in patients with follicular lymphoma and trastuzumab SC in healthy volunteers and patients with early breast cancer have demonstrated substantially shorter administration times and comparable tolerability and pharmacokinetics compared with IV formulations. Rituximab SC 1400-mg and trastuzumab SC 600-mg doses were identified for further study. Phase III clinical data for rituximab SC 1400 mg have shown comparable efficacy to rituximab IV, and initial clinical data suggest comparable efficacy of trastuzumab SC 600 mg and the IV formulation.
Conclusion: Coformulation with rHuPH20 may enable effective, well-tolerated, cost-effective, and convenient SC administration of rituximab and trastuzumab. Additional studies are ongoing.
Trial registration: ClinicalTrials.gov NCT01200758 NCT01292603.
Figures

References
-
- Assouline S, Buccheri V, Delmer A, Doelken G, Gaidano G, McIntyre C, Brewster M, Hourcade-Potelleret F, Sayyed P, Badoux X.2012Subcutaneous rituximab in combination with fludarabine and cyclophosphamide for patients with CLL: initial results of a phase Ib study (SAWYER [BO25341]) show non-inferior pharmacokinetics and comparable safety to that of intravenous rituximab Blood 120: Abstract1637
-
- Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114:230–241. - PubMed
-
- Chan AL, Leung HW, Lu CL, Lin SJ. Cost-effectiveness of trastuzumab as adjuvant therapy for early breast cancer: a systematic review. Ann Pharmacother. 2009;43:296–303. - PubMed
-
- Davies A, Merli F, Mihaljevik B, Siritanaratkul N, Solal-Céligny P, Boehnke A, Berge C, McIntyre C, Barrett M, Macdonald D.2012Pharmacokinetics (PK), safety and overall response rate (ORR) achieved with subcutaneous (SC) administration of rituximab in combination with chemotherapy were comparable to those achieved with intravenous (IV) administration in patients (pts) with follicular lymphoma (FL) in the first-line setting: stage 1 results of the phase III SABRINA study (BO22334) Blood 120: Abstract1629
-
- Del Nagro CJ, Mao CP, Brovarney M.2010Comparison of B cell depletion mediated by intravenous (IV) Rituxan and Rituxan given subcutaneous (SC) in cynomolgus monkeys Blood 116Abstract3980
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

