Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure
- PMID: 24002655
- PMCID: PMC3787132
- DOI: 10.1210/me.2013-1211
Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure
Abstract
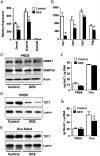
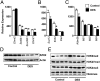
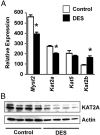
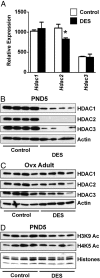
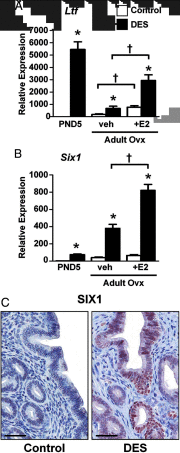
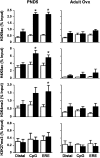
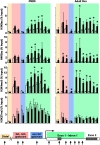
Neonatal exposure to diethylstilbestrol (DES) causes permanent alterations in female reproductive tract gene expression, infertility, and uterine cancer in mice. To determine whether epigenetic mechanisms could explain these phenotypes, we first tested whether DES altered uterine expression of chromatin-modifying proteins. DES treatment significantly reduced expression of methylcytosine dioxygenase TET oncogene family, member 1 (TET1) on postnatal day 5; this decrease was correlated with a subtle decrease in DNA 5-hydroxymethylcytosine in adults. There were also significant reductions in histone methyltransferase enhancer of zeste homolog 2 (EZH2), histone lysine acetyltransferase 2A (KAT2A), and histone deacetylases HDAC1, HDAC2, and HDAC3. Uterine chromatin immunoprecipitation was used to analyze the locus-specific association of modified histones with 2 genes, lactoferrin (Ltf) and sine oculis homeobox 1 (Six1), which are permanently upregulated in adults after neonatal DES treatment. Three histone modifications associated with active transcription, histone H3 lysine 9 acetylation (H3K9ac), H3 lysine 4 trimethylation (H3K4me3), and H4 lysine 5 acetylation (H4K5ac) were enriched at specific Ltf promoter regions after DES treatment, but this enrichment was not maintained in adults. H3K9ac, H4K5ac, and H3K4me3 were enriched at Six1 exon 1 immediately after neonatal DES treatment. As adults, DES-treated mice had greater differences in H4K5ac and H3K4me3 occupancy at Six1 exon 1 and new differences in these histone marks at an upstream region. These findings indicate that neonatal DES exposure temporarily alters expression of multiple chromatin-modifying proteins and persistently alters epigenetic marks in the adult uterus at the Six1 locus, suggesting a mechanism for developmental exposures leading to altered reproductive function and increased cancer risk.
Figures
References
-
- Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

