Preclinical in vivo evaluation of the safety of a multi-shRNA-based gene therapy against HIV-1
- PMID: 24002730
- PMCID: PMC3808742
- DOI: 10.1038/mtna.2013.48
Preclinical in vivo evaluation of the safety of a multi-shRNA-based gene therapy against HIV-1
Abstract
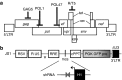
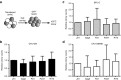
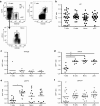
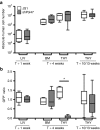
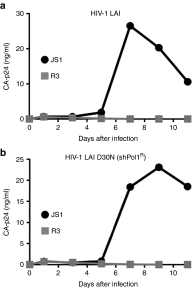
Highly active antiretroviral therapy (HAART) has significantly improved the quality of life and the life expectancy of HIV-infected individuals. Still, drug-induced side effects and emergence of drug-resistant viral variants remain important issues that justify the exploration of alternative therapeutic options. One strategy consists of a gene therapy based on RNA interference to induce the sequence-specific degradation of the HIV-1 RNA genome. We have selected four potent short hairpin RNA (shRNA) candidates targeting the viral capside, integrase, protease and tat/rev open-reading frames and screened the safety of them during human hematopoietic cell development, both in vitro and in vivo. Although the four shRNA candidates appeared to be safe in vitro, one shRNA candidate impaired the in vivo development of the human immune system in Balb/c Rag2(-/-)IL-2Rγc(-/-) (BRG) mice. The three remaining shRNA candidates were combined into one single lentiviral vector (LV), and safety of the shRNA combination during human hematopoietic cell development was confirmed. Overall, we demonstrate here the preclinical in vivo safety of a LV expressing three shRNAs against HIV-1, which is proposed for a future Phase I clinical trial.Molecular Therapy-Nucleic Acids (2013) 2, e120; doi:10.1038/mtna.2013.48; published online 3 September 2013.
Figures


References
-
- Llibre JM, Buzón MJ, Massanella M, Esteve A, Dahl V, Puertas MC, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther (Lond) 2012;17:355–364. - PubMed
-
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. - PubMed
-
- Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. - PubMed
-
- Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5?32/?32 stem cell transplantation. Blood. 2011;117:2791–2799. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

