Successful Desensitisation in a Patient with CRIM-Positive Infantile-Onset Pompe Disease
- PMID: 24002816
- PMCID: PMC3897801
- DOI: 10.1007/8904_2013_250
Successful Desensitisation in a Patient with CRIM-Positive Infantile-Onset Pompe Disease
Abstract
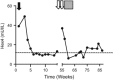
Pompe disease (PD) is a severe life-threatening disease in which enzyme replacement therapy (ERT) with alglucosidase alfa is the only treatment available. Recently it has been shown that antibody formation may have a significant adverse effect on response to ERT. We report a cross-reactive immunologic material (CRIM)-positive PD infant who developed severe infusion-associated reactions (IARs) after 15 uneventful months of ERT. We successfully got the child to tolerate the ERT by a desensitisation protocol. We diluted the total amount of standard alglucosidase alfa infusion (20 mg/kg/dose) to 1/100 (0.2 mg/kg/dose). The original infusion rates were maintained. We doubled this dose every week. No premedication was given. In 8 weeks, we reached the standard dose without any IAR. No further reactions have been observed during 6 months of follow-up. Importantly, clinical deterioration that was observed during the period of reduced enzyme delivery has almost completely reversed. We conclude that this protocol was effective in our patient, while being safe and easy to follow, and may be suitable in selected cases.
Figures
References
-
- Bali DS, Goldstein JL, Banugaria S, et al. Predicting cross-reactive immunological material (CRIM) status in Pompe disease using GAA mutations: lessons learned from 10 years of clinical laboratory testing experience. Am J Med Genet C Semin Med Genet. 2012;160:40–49. doi: 10.1002/ajmg.c.31319. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources