Integrin α3β1 can function to promote spontaneous metastasis and lung colonization of invasive breast carcinoma
- PMID: 24002891
- PMCID: PMC3947021
- DOI: 10.1158/1541-7786.MCR-13-0184
Integrin α3β1 can function to promote spontaneous metastasis and lung colonization of invasive breast carcinoma
Abstract
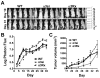
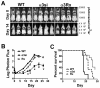
Significant evidence implicates α3β1 integrin in promoting breast cancer tumorigenesis and metastasis-associated cell behaviors in vitro and in vivo. However, the extent to which α3β1 is actually required for breast cancer metastasis remains to be determined. We used RNA interference to silence α3 integrin expression by approximately 70% in 4T1 murine mammary carcinoma cells, a model of aggressive, metastatic breast cancer. Loss of α3 integrin reduced adhesion, spreading, and proliferation on laminin isoforms, and modestly reduced the growth of orthotopically implanted cells. However, spontaneous metastasis to lung was strikingly curtailed. Experimental lung colonization after tail vein injection revealed a similar loss of metastatic capacity for the α3-silenced (α3si) cells, suggesting that critical, α3-dependent events at the metastatic site could account for much of α3β1's contribution to metastasis in this model. Reexpressing α3 in the α3si cells reversed the loss of metastatic capacity, and silencing another target, the small GTPase RhoC, had no effect, supporting the specificity of the effect of silencing α3. Parental, α3si, and α3-rescued cells, all secreted abundant laminin α5 (LAMA5), an α3β1 integrin ligand, suggesting that loss of α3 integrin might disrupt an autocrine loop that could function to sustain metastatic growth. Analysis of human breast cancer cases revealed reduced survival in cases where α3 integrin and LAMA5 are both overexpressed.
Implications: α3 integrin or downstream effectors may be potential therapeutic targets in disseminated breast cancers, especially when laminin α5 or other α3 integrin ligands are also over-expressed.
©2013 AACR.
Figures

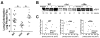



Similar articles
-
Integrin α3β1 regulates tumor cell responses to stromal cells and can function to suppress prostate cancer metastatic colonization.Clin Exp Metastasis. 2013 Apr;30(4):541-52. doi: 10.1007/s10585-012-9558-1. Epub 2012 Dec 6. Clin Exp Metastasis. 2013. PMID: 23224938 Free PMC article.
-
Comparative use of CRISPR and RNAi to modulate integrin α3β1 in triple negative breast cancer cells reveals that some pro-invasive/pro-metastatic α3β1 functions are independent of global regulation of the transcriptome.PLoS One. 2021 Jul 16;16(7):e0254714. doi: 10.1371/journal.pone.0254714. eCollection 2021. PLoS One. 2021. PMID: 34270616 Free PMC article.
-
The CD9/CD81 tetraspanin complex and tetraspanin CD151 regulate α3β1 integrin-dependent tumor cell behaviors by overlapping but distinct mechanisms.PLoS One. 2013 Apr 17;8(4):e61834. doi: 10.1371/journal.pone.0061834. Print 2013. PLoS One. 2013. PMID: 23613949 Free PMC article.
-
Integrin α3β1 as a breast cancer target.Expert Opin Ther Targets. 2011 Oct;15(10):1197-210. doi: 10.1517/14728222.2011.609557. Epub 2011 Aug 13. Expert Opin Ther Targets. 2011. PMID: 21838596 Free PMC article. Review.
-
Cancer Cell-derived Secretory Factors in Breast Cancer-associated Lung Metastasis: Their Mechanism and Future Prospects.Curr Cancer Drug Targets. 2020;20(3):168-186. doi: 10.2174/1568009620666191220151856. Curr Cancer Drug Targets. 2020. PMID: 31858911 Free PMC article. Review.
Cited by
-
Tetraspanin CD9 determines invasiveness and tumorigenicity of human breast cancer cells.Oncotarget. 2015 Apr 10;6(10):7970-91. doi: 10.18632/oncotarget.3419. Oncotarget. 2015. PMID: 25762645 Free PMC article.
-
High integrin α3 expression is associated with poor prognosis in patients with non-small cell lung cancer.Transl Lung Cancer Res. 2020 Aug;9(4):1361-1378. doi: 10.21037/tlcr-19-633. Transl Lung Cancer Res. 2020. PMID: 32953510 Free PMC article.
-
Interplay of integrins and selectins in metastasis.Mol Oncol. 2025 Jun;19(6):1582-1611. doi: 10.1002/1878-0261.70026. Epub 2025 May 6. Mol Oncol. 2025. PMID: 40327521 Free PMC article. Review.
-
Pathogenicity of Bovine Neonatal Pancytopenia-associated vaccine-induced alloantibodies correlates with Major Histocompatibility Complex class I expression.Sci Rep. 2015 Aug 3;5:12748. doi: 10.1038/srep12748. Sci Rep. 2015. PMID: 26235972 Free PMC article.
-
Elucidation of the Roles of Tumor Integrin β1 in the Extravasation Stage of the Metastasis Cascade.Cancer Res. 2016 May 1;76(9):2513-24. doi: 10.1158/0008-5472.CAN-15-1325. Epub 2016 Mar 17. Cancer Res. 2016. PMID: 26988988 Free PMC article.
References
-
- Plopper GE, Domanico SZ, Cirulli V, Kiosses WB, Quaranta V. Migration of breast epithelial cells on Laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res Treat. 1998;51:57–69. - PubMed
-
- Henning K, Berndt A, Katenkamp D, Kosmehl H. Loss of laminin-5 in the epithelium-stroma interface: an immunohistochemical marker of malignancy in epithelial lesions of the breast. Histopathology. 1999;34:305–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

