Investigations of two bidirectional carbon monoxide dehydrogenases from Carboxydothermus hydrogenoformans by protein film electrochemistry
- PMID: 24002936
- PMCID: PMC3955222
- DOI: 10.1002/cbic.201300270
Investigations of two bidirectional carbon monoxide dehydrogenases from Carboxydothermus hydrogenoformans by protein film electrochemistry
Abstract
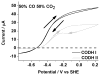
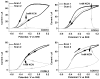
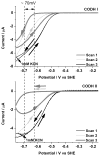
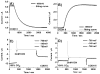
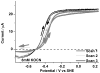
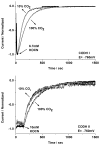
Carbon monoxide dehydrogenases (CODHs) catalyse the reversible conversion between CO and CO2 . Several small molecules or ions are inhibitors and probes for different oxidation states of the unusual [Ni-4 Fe-4 S] cluster that forms the active site. The actions of these small probes on two enzymes-CODH ICh and CODH IICh -produced by Carboxydothermus hydrogenoformans have been studied by protein film voltammetry to compare their behaviour and to establish general characteristics. Whereas CODH ICh is, so far, the better studied of the two isozymes in terms of its electrocatalytic properties, it is CODH IICh that has been characterised by X-ray crystallography. The two isozymes, which share 58.3% sequence identity and 73.9% sequence similarity, show similar patterns of behaviour with regard to selective inhibition of CO2 reduction by CO (product) and cyanate, potent and selective inhibition of CO oxidation by cyanide, and the action of sulfide, which promotes oxidative inactivation of the enzyme. For both isozymes, rates of binding of substrate analogues CN(-) (for CO) and NCO(-) (for CO2 ) are orders of magnitude lower than turnover, a feature that is clearly revealed through hysteresis of cyclic voltammetry. Inhibition by CN(-) and CO is much stronger for CODH IICh than for CODH ICh, a property that has relevance for applying these enzymes as model catalysts in solar-driven CO2 reduction.
Keywords: CO2 reduction; carbon monoxide dehydrogenases; cyclic voltammetry; inhibitors; protein film electrochemistry.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

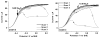
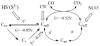
References
-
- Meyer O, Schlegel HG. Annu Rev Microbiol. 1983;37:277–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/H003878/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 GM039451/GM/NIGMS NIH HHS/United States
- H003878-1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- GM39451/GM/NIGMS NIH HHS/United States
- BB/I022309/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

