Single transcriptional and translational preQ1 riboswitches adopt similar pre-folded ensembles that follow distinct folding pathways into the same ligand-bound structure
- PMID: 24003028
- PMCID: PMC3905878
- DOI: 10.1093/nar/gkt798
Single transcriptional and translational preQ1 riboswitches adopt similar pre-folded ensembles that follow distinct folding pathways into the same ligand-bound structure
Abstract
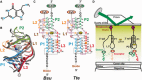
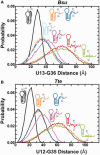
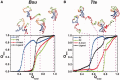
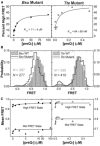
Riboswitches are structural elements in the 5' untranslated regions of many bacterial messenger RNAs that regulate gene expression in response to changing metabolite concentrations by inhibition of either transcription or translation initiation. The preQ1 (7-aminomethyl-7-deazaguanine) riboswitch family comprises some of the smallest metabolite sensing RNAs found in nature. Once ligand-bound, the transcriptional Bacillus subtilis and translational Thermoanaerobacter tengcongensis preQ1 riboswitch aptamers are structurally similar RNA pseudoknots; yet, prior structural studies have characterized their ligand-free conformations as largely unfolded and folded, respectively. In contrast, through single molecule observation, we now show that, at near-physiological Mg(2+) concentration and pH, both ligand-free aptamers adopt similar pre-folded state ensembles that differ in their ligand-mediated folding. Structure-based Gō-model simulations of the two aptamers suggest that the ligand binds late (Bacillus subtilis) and early (Thermoanaerobacter tengcongensis) relative to pseudoknot folding, leading to the proposal that the principal distinction between the two riboswitches lies in their relative tendencies to fold via mechanisms of conformational selection and induced fit, respectively. These mechanistic insights are put to the test by rationally designing a single nucleotide swap distal from the ligand binding pocket that we find to predictably control the aptamers' pre-folded states and their ligand binding affinities.
Figures


References
-
- Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. - PubMed
-
- Nudler E, Mironov AS. The riboswitch control of bacterial metabolism. Trends Biochem. Sci. 2004;29:11–17. - PubMed
-
- Mandal M, Breaker RR. Gene regulation by riboswitches. Nat. Rev. Mol. Cell Biol. 2004;5:451–463. - PubMed
-
- Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu. Rev. Microbiol. 2005;59:487–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

