How minute sooglossid frogs hear without a middle ear
- PMID: 24003145
- PMCID: PMC3780892
- DOI: 10.1073/pnas.1302218110
How minute sooglossid frogs hear without a middle ear
Abstract
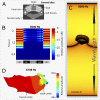
Acoustic communication is widespread in animals. According to the sensory drive hypothesis [Endler JA (1993) Philos Trans R Soc Lond B Biol Sci 340(1292):215-225], communication signals and perceptual systems have coevolved. A clear illustration of this is the evolution of the tetrapod middle ear, adapted to life on land. Here we report the discovery of a bone conduction-mediated stimulation of the ear by wave propagation in Sechellophryne gardineri, one of the world's smallest terrestrial tetrapods, which lacks a middle ear yet produces acoustic signals. Based on X-ray synchrotron holotomography, we measured the biomechanical properties of the otic tissues and modeled the acoustic propagation. Our models show how bone conduction enhanced by the resonating role of the mouth allows these seemingly deaf frogs to communicate effectively without a middle ear.
Keywords: X-ray imaging; audition; earless frog; extra-tympanic pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Allen J. Cochlear modeling. IEEE ASSP Mag. 1985;2(1):3–20.
-
- Clack JA. Discovery of the earliest-known tetrapod stapes. Nature. 1989;342(6248):425–427. - PubMed
-
- Van Bergeijk WA. Evolution of the sense of hearing in vertebrates. Am Zool. 1966;6(3):371–377. - PubMed
-
- Jaslow AP, Hetherington TE, Lombard RE. In: The Evolution of the Amphibian Auditory System. Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. New York: Wiley; 1988. pp. 69–91.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

