Mitochondrial regulation of cell death
- PMID: 24003207
- PMCID: PMC3753705
- DOI: 10.1101/cshperspect.a008706
Mitochondrial regulation of cell death
Abstract
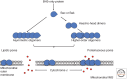
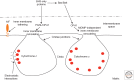
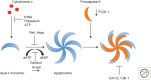
Although required for life, paradoxically, mitochondria are often essential for initiating apoptotic cell death. Mitochondria regulate caspase activation and cell death through an event termed mitochondrial outer membrane permeabilization (MOMP); this leads to the release of various mitochondrial intermembrane space proteins that activate caspases, resulting in apoptosis. MOMP is often considered a point of no return because it typically leads to cell death, even in the absence of caspase activity. Because of this pivotal role in deciding cell fate, deregulation of MOMP impacts on many diseases and represents a fruitful site for therapeutic intervention. Here we discuss the mechanisms underlying mitochondrial permeabilization and how this key event leads to cell death through caspase-dependent and -independent means. We then proceed to explore how the release of mitochondrial proteins may be regulated following MOMP. Finally, we discuss mechanisms that enable cells sometimes to survive MOMP, allowing them, in essence, to return from the point of no return.
Figures

References
-
- Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K 2007. Mitochondrial disruption in Drosophila apoptosis. Dev Cell 12: 793–806 - PubMed
-
- Allan LA, Clarke PR 2007. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 26: 301–310 - PubMed
-
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR 2003. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 5: 647–654 - PubMed
-
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, et al. 1997. Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
